5: Strategies in Disparlure Synthesis
- Page ID
- 21839
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The gypsy moth (Porthytria dispar) is a serious pest of the forests. In 1976 B.A. Bierl et.al., (Science, 170,88 (1970)) isolated the sex pheromone from extracts of 78,000 tips of the last two abdominal segments of female moths. The structure was assigned as 5.1. Later, the precursor molecule – the cis-olefin was also isolated from the same source.
A laboratory bioassay from synthetic materials showed that just 2 pg of 5.1 was enough to elicit bioactivity. Since the availability of the molecule from natural sources was very minute even for structure elucidation problems and study its anticipated role as pest control molecule, there was intense interest in an efficient synthesis of this molecule. Some disconnections for this simple molecule are depicted in Figure 5.2.
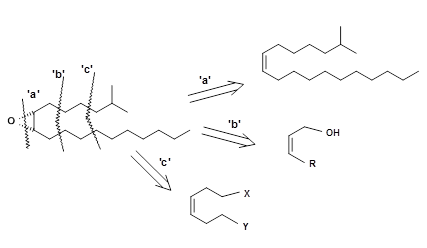
Epoxides could be made from corresponding olefins. In this case, the olefin should be Z-olefin. When synthesis of such olefins are not stereospecific, direct epoxidation using peroxides would yield a mixture of α- and β-epoxide from both isomeric olefins. To avoid such mixtures at the last stage, one should introduce selectivity at an early stage of the synthesis.
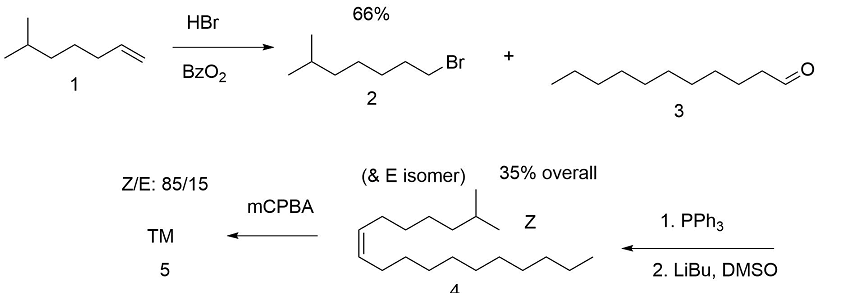
The first attempt was directed towards synthesis of the appropriate olefin and epoxidation (B.A. Bierl et.al., (Science, 170, 88 (1970)). The stereoselectivity was unsatisfactory (Fig 5.3). This necessitated extensive purification.
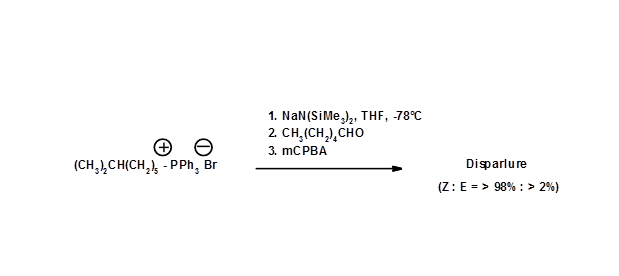
The ratio of cis- / trans- isomers in Wittig Olefination reaction could be altered by modification of reagents and reaction parameters. H.T. Bestmann et.al., (Chem. Ber., 109, 3375 (1976)) were able to improve the synthesis by modifying the Wittig reaction conditions (Fig 5.4).
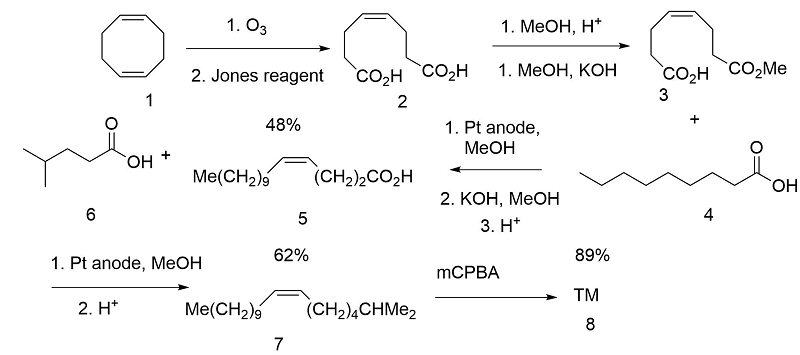
Pure cis- olefins could be obtained by catalytic reduction of acetylenes (Angew. Chem., Int. Ed., 11, 60 (1972). Klunenberg et.al., (Angew. Chem., Int. Ed., 17, 47 (1978) took advantage of the cis- olefin moieties in 1,5-cyclooctadiene by selective oxidation of one double bond. The chains were introduced by sequential Kolbe electrolysis (Fig 5.5).
Synthesis of Optically pure Disparlures
Epoxidation of olefins yield only racemates unless the epoxidation step involves an asymmetric synthesis. Synthesis of pure (+)- and (-)- isomers could be achieved in three ways.
- Resolution of a racemate: This could be a method of choice when both enantiomers are needed for SAR studies. All antipodes of the compound would be available through identical synthetic pathways.
- Asymmetric synthesis of appropriate intermediates: When only one of the antipodes is desired, this process provides a wide range of synthetic possibilities for investigation. When a large number of closely related compounds are the targets, this method is a better choice.
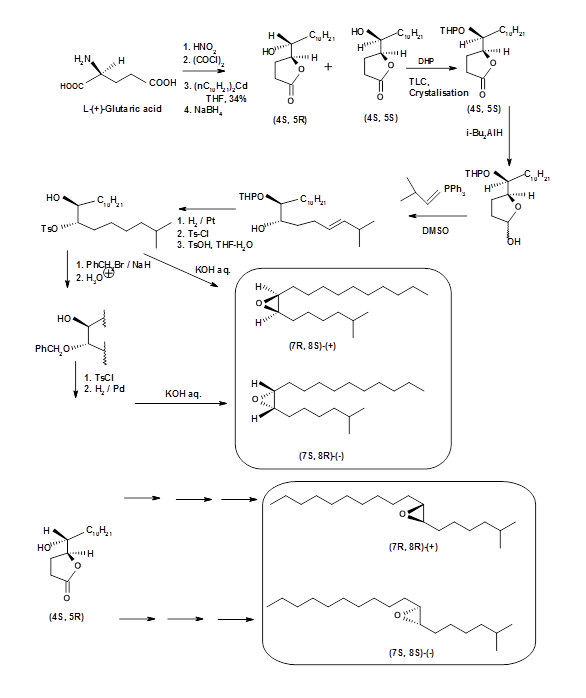
- Sourcing the chiral intermediate from a suitable chiral pool: Once the chiral target is clear, this could be a method of choice. In the case of Disparlure, the amount that could be isolated from gypsy moth was so small that even the optical rotation could not be determined. Several workers have reported synthesis of optically pure (+)- and (-)- Disparlure and its structural isomers. The first report came from S. Iwaki et.al., (J. Am. Chem. Soc., 96, 7842 (1974)). They started with L-(+)-Glutamic acid and resolved the intermediate diastereomeric alcohol-lactones by repeated crystallization technique (Fig 5.6). Their synthesis was not stereospecific. SAR studies revealed that the cis-(+)- isomer was most effective.
Mori et.al.,( Tet. Lett., 3953 (1976); Tetrahedron, 53, 833 (1979)) soon followed with a synthesis of (+)- and (-)- Disparlures starting from L-(+)-tartaric acid (Fig 5.7).
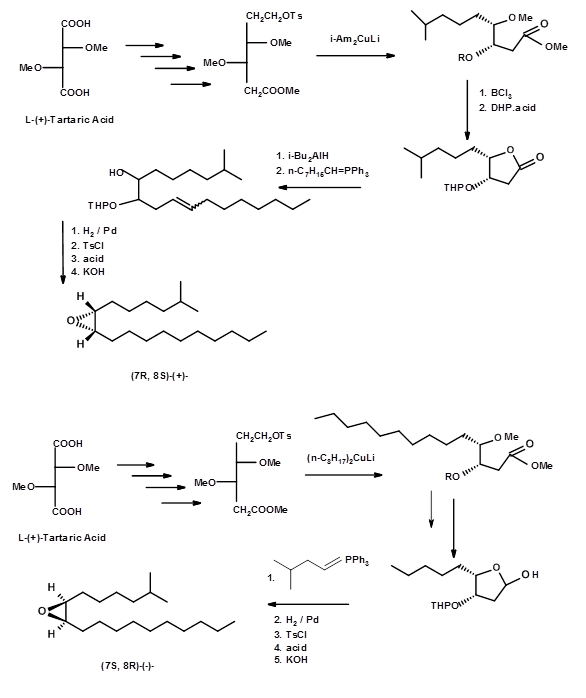
This synthesis had the merit that some of the chiral intermediated were crystallisable and therefore amenable for easy purification to very pure intermediates and pure final products. Their intermediates were subjected to critical spectral analysis to assess their purity. Thus, their bio-assays gave more reliable data. A synthesis of (+)- and (-)- Disparlure from another chiral synthon - isopropylidene D- and L- erythroses - was reported by Alexandros E. Koumbis et.al., (Tetrahedron Letters, 46, 4353 (2005)) (Fig 5.8).

A successful synthesis of (+)- Disparlure by the application of Sharpless epoxidation was reported by Kossier B. E.. et.al., (J. Am. Chem. Soc., 103, 464 (1981)) (Fig 5.9).
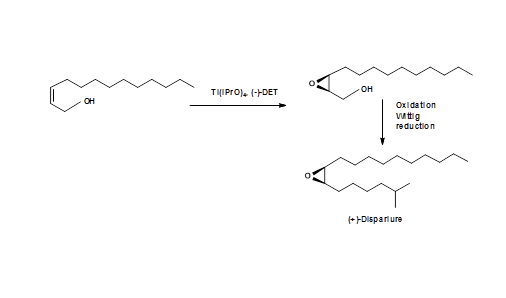
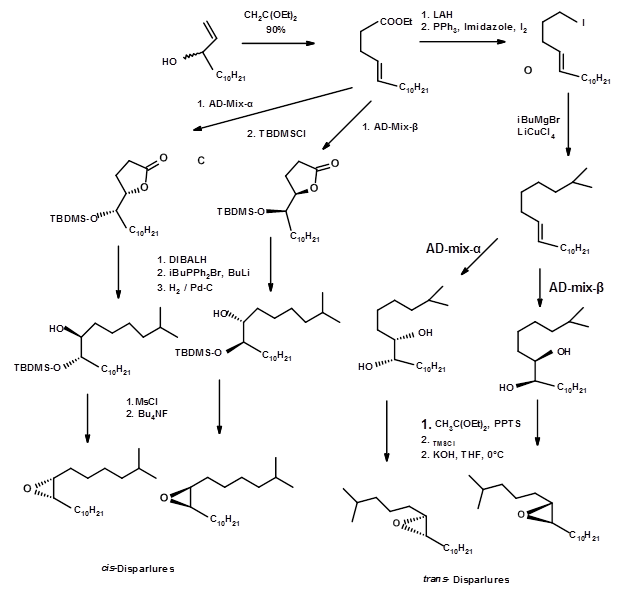
Synthesis of all four isomers in a very pure form came from the school of Sharpless E. B. ( Tet. Lett., 6411 (1992)). Using both the chiral hydroxylation agents, they reported an efficient synthesis of all enantiomers (Fig 5.10). The efficiency of the asymmetric synthesis was as high as 95% and gave 100% pure intermediated by crystallization. The overall process was very efficient.