III. Intramolecular Addition (Cyclization) Reactions
- Page ID
- 23952
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)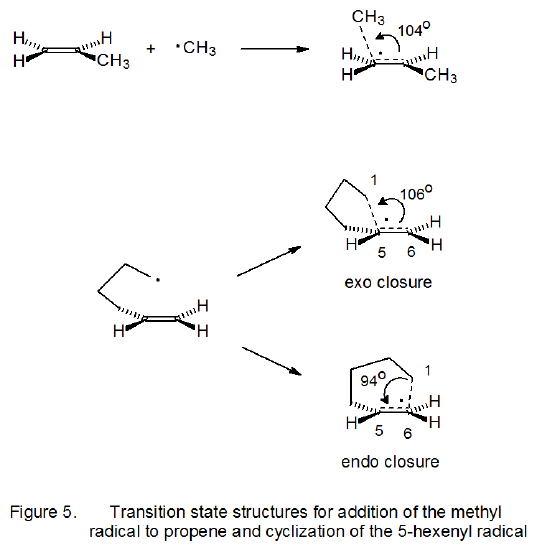
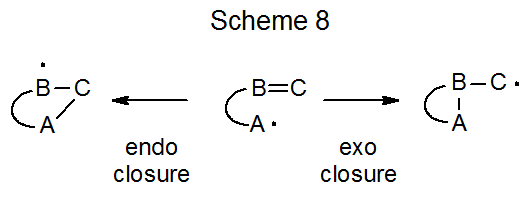
In addition to describing cyclization reactions by the size of the ring produced, the terms exo and endo indicate the way in which the ring is formed. The meaning of these terms is illustrated in the reactions shown is Scheme 8. When the exo/endo terminology is used to describe ring formation from reaction of the 5-hexenyl radical, the five-membered ring is seen as arising from exo closure and the six-membered one from endo closure (Scheme 7).
2. Transition-State Structure
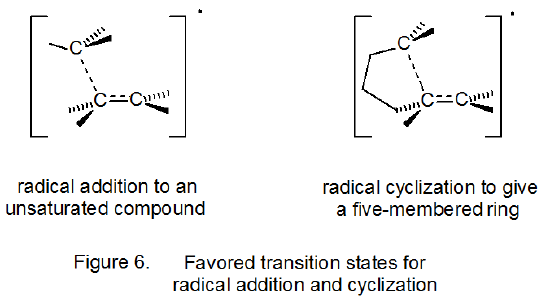
Transition-state structures for radical addition and radical cyclization are given in Figure 6 in a general form. For cyclization reactions not only ring size but also ring conformation affect transition-state energy; thus, both chair-like25,27 and boat-like27,30 structures are possible during five-membered ring formation. For the unsubstituted 5-hexenyl radical the chair-like transition state leading to a five-membered ring is calculated to be lower in energy, but only slightly so, than the boat-like transition state (Scheme 9).27 (The “flagpole” interactions that contribute to making the boat conformation of cyclohexane much less stable than the chair conformation are less severe in the boat-like transition state for radical cyclization.) Both transition states (boat-like and chair-like) leading to a five-membered ring (Scheme 9) are calculated to be lower in energy than any transition states leading to a six-membered ring. These calculations match well the experimental observation that cyclization of the 5-hexenyl radical gives a five-membered ring in a highly regioselective fashion (eq 14, R = H).25,31

.png?revision=1&size=bestfit&width=360&height=155)
3. Altering Normal Regioselectivity
a. Steric Interactions and Adduct-Radical Stability
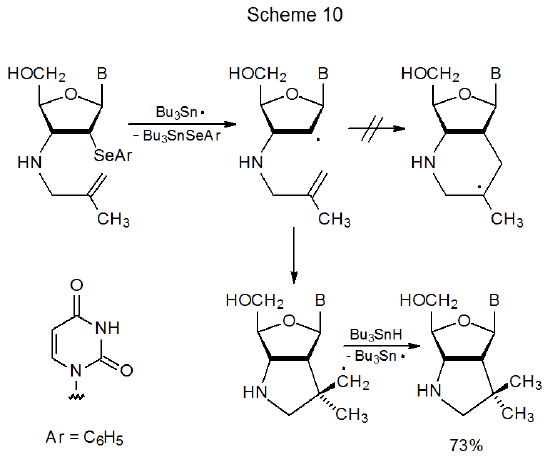
Although ring size is the primary factor affecting regioselectivity in cyclization reactions, other factors sometimes have a modifying effect; for example, in the reaction of the 5-methyl-5-hexenyl radical the presence of the methyl group increases the amount of six-membered-ring formation (eq 14, R = CH3).25,27 In this reaction steric effects and adduct-radical stability both favor a six-membered ring. The transition state in this reaction presumably is reached late enough that either steric effects or adduct-radical stability or both have a substantial impact on regioselectivity. Predicting when the transition state in this type of reaction will be early enough to cause highly regioselective, five-membered-ring formation is not easy. In the reaction shown in Scheme 10, where steric interactions and adduct-radical stability favor six-membered-ring formation at least as much as they do in the reaction shown in eq 14 (R=CH3), only a product with a five-membered ring forms.32
b. Thermodynamic Control
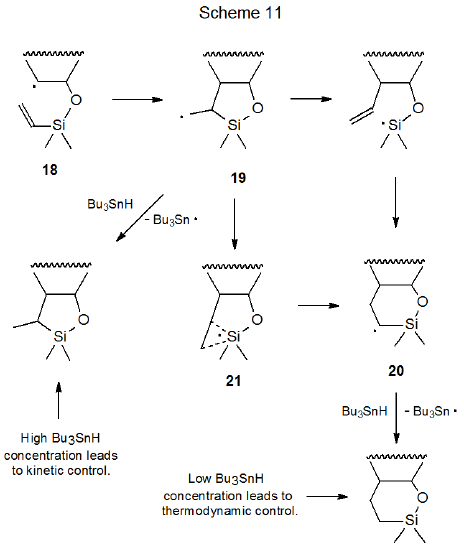
Although kinetically controlled reaction is the norm in radical cyclization, thermodynamic control is observed in the reaction shown in eq 15 where the substrate is an unsaturated silyl ether and the hydrogen donor (Bu3SnH) is maintained at a low level.33 When this reaction is conducted with a high Bu3SnH concentration, kinetically controlled, five-membered ring formation is the major reaction pathway. An explanation for this dependence on hydrogen-donor concentration begins with the radical 18 cyclizing to form 19, a radical with a new five-membered ring (Scheme 11). If the concentration of Bu3SnH is high, hydrogen-atom abstraction rapidly completes the reaction, but if the donor concentration is low, rearrangement to the more stable radical 20, via the transition state 21, takes place before hydrogen-atom abstraction can occur.34 Hydrogen-atom abstraction by 20 then gives the thermodynamically favored product. An alternative mechanism for this reaction, also shown in Scheme 11, is that ring opening of 19 produces a silicon-centered radical that undergoes ring closure to give the intermediate radical 20.35
.png?revision=1&size=bestfit&width=415&height=185)
c. Reversal Due to Stereochemistry
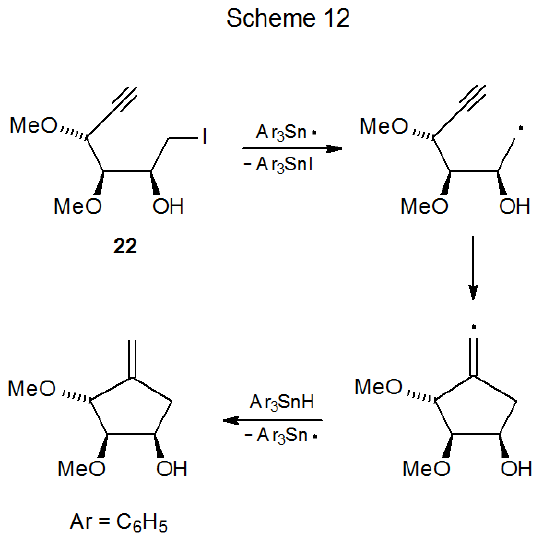
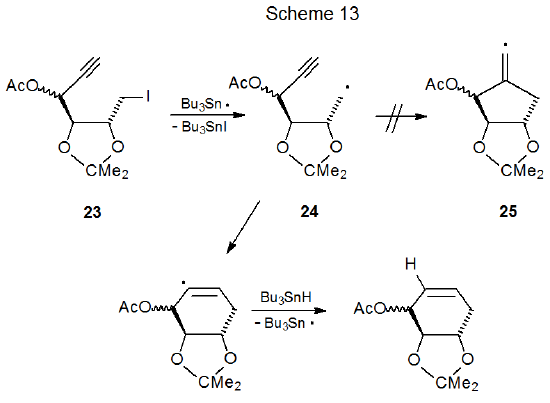
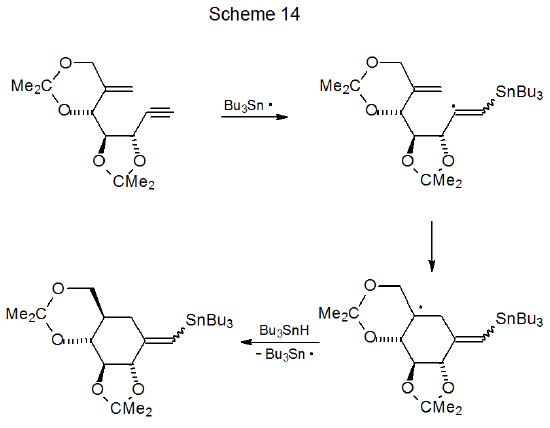
One situation where six-membered ring formation is favored consistently over reaction producing a five-membered ring is when cyclization would produce a pair of trans-fused, five-membered rings. Reactions of iodides 22 and 23 illustrate the effect that stereochemistry can have on radical cyclization. The acyclic iodide 22 undergoes an expected cyclization to give a five-membered ring (Scheme 12),36 but reaction of the iodide 23 forms a six-membered ring (Scheme 13).37 Since a trans fusion between two five-membered rings would produce the highly strained radical 25, the stereochemistry of the radical 24 dictates the regioselectivity of the cyclization reaction. Six-membered-ring formation also occurs in the reaction shown in Scheme 1438, again, because the other option would force the formation of trans-fused, five-membered rings.