1.19: Amines- Structure and Synthesis
- Page ID
- 81785
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Structural Types
Last time we completed our study of carbohydrates. Now we are turning our attention to another important class of organic compounds, amines. Many important drugs are amines, the bases present in RNA and DNA are amines, and the fundamental building blocks of proteins are amino acids.
The functional group of an amine is the nitrogen atom connected by three sigma bonds to alkyl groups or hydrogen atoms. (Aryl groups - benzene rings - can also be connected to a nitrogen in amines, but we will not study these until later in the course.) The chemistry of amides is different enough from that of amines that they are normally not included among the amines. The nitrogen atom of an amine can also be included in a ring. Such amines are called "heterocycles." (Recall that nitrogen is a heteroatom -- not a carbon or a hydrogen.)
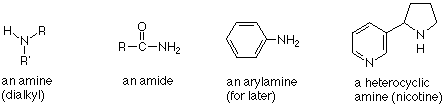
Amines are classified as primary, secondary, or tertiary according to the number of carbons bonded directly to the nitrogen atom. Primary amines have one carbon bonded to the nitrogen. Secondary amines have two carbons bonded to the nitrogen, and tertiary amines have three carbons bonded to the nitrogen. The system is superficially similar to the way we have classified alcohols, but the important difference is that in alcohols we were counting bonds to the carbon which carried the OH group. For amines, we are counting the carbons bonded to the nitrogen.
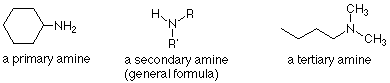
Since nitrogen has a normal valence of three, we can also conclude that there are two N-H bonds in primary amines and one N-H bond in secondary amines. In tertiary amines there are no N-H bonds.
Bonding, Shape and Hybridization
Now, let's look at the bonding around the nitrogen atom of an amine. First, we need to remember that the nitrogen, in addition to forming three sigma bonds, also carries an unshared electron pair. This means that there are four groups of electrons associated with the nitrogen. Mutual repulsion of these groups leads to a tetrahedral arrangment, much like that of a typical sp3 carbon atom. This predicts a bond angle between the N-H bonds of 109.5o which agrees pretty well with the observed value of 107o found in ammonia (which is the "smallest" amine, like water is the smallest alcohol).
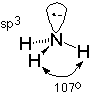
Since the bonds connected to the nitrogen are shaped like a flattened pyramid, the arrangement is often called pyramidal. This ignores the unshared electron pair, whose inclusion leads to the tetrahedral description and the corresponding understanding of the nitrogen's hybridization as sp3. The example used to illustrate this is ammonia, but the nitrogen is also well described as having sp3 hybridization primary, secondary and tertiary amines as well.
Hydrogen Bonds
The N-H bonds in amines are somewhat polar. As we might guess from considering electronegativities (estimated from positions in the periodic table), the N-H bond is more polar than the C-H bond and less polar than the O-H bond. This polarity shows up in a comparision of physical properties of amines and alcohols.
The simplest examples are water and ammonia. Water boils at 100oC, while ammonia boils at -33oC. This is interpreted to mean that it takes a good deal more energy to boil water than ammonia. Correspondingly, the forces between molecules of water (which resist separating them in the boiling process) are much stronger than those between molecules of ammonia. The most important of these forces is called the hydrogen bond.
The hydrogen bond is much weaker than a covalent bond. Breaking a hydrogen bond requires about 10% of the energy required to break a typical covalent bond. This is consistent with the fact that we can boil water without breaking any covalent bonds (the water molecules remain intact). Separation of one molecule from another only requires breaking the weaker hydrogen bonds.
One useful picture of a hydrogen bond is electrostatic -- an attraction between the positive end of a dipole on one molecule and the negative end of a dipole on another. The more polar the molecules, the greater the degree of positive and negative charge associated with the dipoles, and the stronger the hydrogen bonds. In ammonia and water, the most concentrated and available negatively charged region is where the unshared electron pair is. The positively charged regions are the hydrogen ends of the N-H bonds. We can envision the hydrogen bond as a weak (compared to covalent bonds) attraction between the unshared electron pairs on of a nitrogen in one molecule and a hydrogen (covalently bonded to nitrogen) on another.
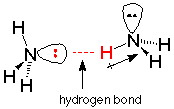
Hydrogen bonds are extremely important in the chemistry of the genetic code. As we will study later, the double strands of DNA are held together by hydrogen bonds. The replication of DNA depends on hydrogen bonds which selectively connect specific base pairs, as do the several steps by which the genetic message determines the specific order of amino acids in a protein.
Amines as Bases
The distinguishing chemical property of amines is that they are bases. This is a direct consequence of the presence of the unshared electron pair on the nitrogen, which makes them Lewis bases. The basic unshared electron pair is less tightly held by the nitrogen of an amine than the corresponding oxygen of an alcohol, which makes it more available to act as a base. Consequently amines (and ammonia) are more basic than alcohols (and water), and less basic than alkoxide (RO-) and hydroxide (OH-) ions. It is convenient to think about the base strength of amines and ammonia in terms of the pKa of their conjugate acids, the ammonium ions. These ideas can be summarized in the following scheme, where we use water and ammonia as stand-ins for alcohols and amines:
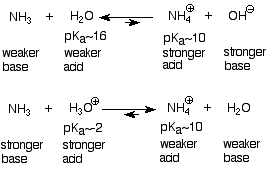
As we have come to expect, these reactions go in the direction which consumes the stronger bases and acids and generates the weaker ones. Ammonium ions (pKa ~10) are stronger acids than water (pKa ~16, so water is produced when an ammonium ion is treated with hydroxide ion. Ammonium ions (pKa ~10) are weaker acids than H3O+ (pKa ~-2), so they are produced when amines are treated with aqueous solutions of strong mineral acids like sulfuric or hydrochloric acids. If the water is removed, there remains an ammonium salt which incorporates the negative counterion of the mineral acid (sulfate or chloride).
Since the basic properties of amines arise from the presence of the unshared electron pair on nitrogen, the strengths of primary, secondary and tertiary amines are quite similar. Aryl amines (those where the nitrogen is connected directly to an aromatic amine) weaker bases, but we will take that topic up later in the course.
Synthesis of Amines
Our last topic for today is the synthesis of amines. While it is possible to make alkyl amines (an example which is a primary amine with a primary alkyl group) would be RCH2NH2) by reaction of a primary halide with ammonia, these reactions are seldom very practical.
The more practical approches to making alkyl amines involve reactions which are reductions. The first of these is a reaction of an amide with lithium aluminum hydride. The overall effect of this reaction is to replace the carbonyl oxygen of the amide with two hydrogens from lithium aluminum hydride. We will not discuss the mechanism, but it is likely that it begins with the familiar nucleophilic attack of the hydride (H:- of lithium aluminum hydride on the carbonyl carbon of the amide. (This reaction is discussed in Section 13.8B of Brown.)
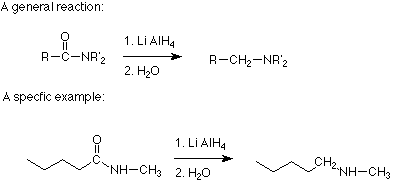
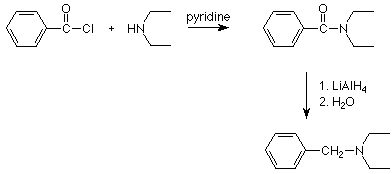
Notice that any other atoms or alkyl groups attached to the amide nitrogen are not changed. Since amides are commonly made by the reaction of acid chlorides with an amine, the two step sequence which begins with reacting an amine (or ammonia) with an acid chloride and follows with reduction of the amide using lithium aluminum hydride results in the substitution of an alkyl group on the nitrogen. Here's an example:
Another reductive synthesis of amines reacts a nitrile (RCN) with hydrogen in the presence of a catalyst which is very finely divided nickel. This reaction adds two molecules of H2 to the triple bond of the nitrile, and produces a primary alkyl primary amine (RCH2NH2. Lithium aluminum hydride will also carry out this reduction.
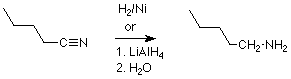
Recall that nitriles are commonly made by the reaction of primary alkyl halides with sodium or potassium cyanide, and we again have a two step sequence as in this example:
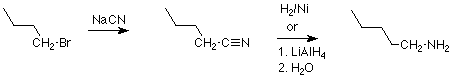
Finally, there is also a reductive method which involves a ketone or aldehyde carbonyl reaction. Early in the course we studied a reaction between primary amines and ketones or aldehydes which resulted in the replacement of the carbonyl oxygen by the nitrogen of the amine. Water was eliminated and the product was an imine.
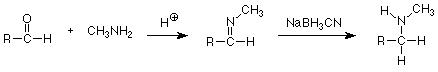
If this reaction is carried out in a solution containing sodium cyanoborohydride (NaBH3CN, a less reactive analog of sodium borohydride) the imine is reduced to an amine even though there is also a reducible carbonyl group in the reaction mixture. The formation of glutamic acid (from which the amino groups of all other amino acids arise by transamination) is a biological analog of this "reductive amination." Here the reducing agent is NADPH+, one of several reducing agents which are common in biochemical reactions.
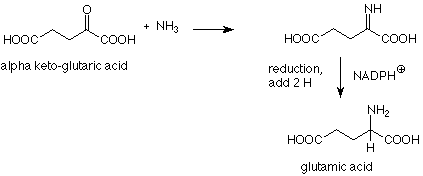
Again, we see that biochemical reactions use organic reaction mechanisms.
Contributors
Kirk McMichael (Washington State University)


