1.28: Polymers
- Page ID
- 81794
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Polymers - Structure and Response to Heat
Let's begin by noticing some important real-world characteristics of polymers. While they all contain molecules with very long chains, there are some important differences between the properties of different types of polymers. Most polymers are formed into the desired shapes after softening or melting by heating. Some, like the familiar polyethylene and polystyrene, may be melted and reshaped again and again. These are called thermoplastic polymers.
Others char or burn when reheated. These are called thermosetting polymers. Examples include Bakelite and vulcanized rubber. The structural difference between these polymers is that the thermosetting polymers have crosslinks between the chains and the thermoplastic polymers do not. When a thermoplastic polymer is heated the chains are free to move past each other making the sample less rigid and eventually melting it. This cannot happen with a thermosetting polymer, since its chains are locked together by the cross links. The energy from the heat must eventually go into breaking bonds which leads to decomposition of the polymer.
This schematic view suggests the difference:
We noticed crosslinking earlier when we saw how the disulfide crosslink formed by oxidation of the SH group in cysteine was important in maintaining protein structure.
Repeating Units and Monomers
Since polymers are made by linking together many identical small molecules, there are repeating units in polymers. Here's an example, polyvinyl chloride, in which the repeating unit is -CH2-CHCl-.
In poly(vinyl chloride) the repeating unit comes directly from the end-to-end linking of many vinyl chloride molecules. A molecule from which a polymer is made is called a monomer. Each vinyl chloride monomer molecule contributes a CH2 group joined to a CHCl unit by a single bond. This single bond is a remnant of the double bond which joined those groups in the vinyl chloride molecule. This is just what happens in an addition reaction of an alkene. We'll see how an addition reaction leads to such polymers in a few paragraphs.
Repeating units can also be made from two monomers. Here's an example:
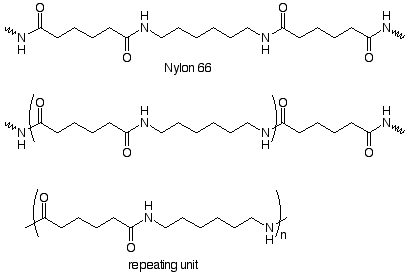
We notice that the repeating unit is linked to the rest of the chain by amide functional groups, and that the repeating unit contains an amide group. We can deduce the structrure of the monomers by imagining the compounds which might be used to make the amide group. In the industrial process these are a dicarboxylic acid (adipic acid) and a diamine (1,6-hexanediamine). These form a salt when dissoved in alcohol. The salt is heated at 250o under pressure to form the amide bonds. Nylon 66 is the result.
Step-Growth Polymers
Polymers formed in this way, where both ends of the growing chain have functional groups which can react with a monomer or with the appropriate functional group on another chain, are called step-growth polymers. Let's look at another step-growth polymer, but this time we'll look at the monomers and propose a structure for the polymer that would result.
The monomers are a dicarboxylic acid (terephthalic acid) and a dialcohol, also called a diol (ethylene glycol). We immediately recognize that these monomers can make an ester, so that we expect our polymer to be linked by ester functional groups. The resulting polymer is called polyethylene terephthalate and is the common polyester of plastic pop bottles and polyester fabrics.
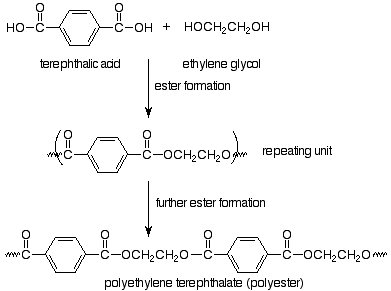
Notice that in choosing how to represent the repeating unit in step-growth polymers we have picked the particular repeating unit (out of several possibilities) which is linked to the rest of the polymer through functional group bonds. In the case of Nylon, the functional group bond is an amide bond, so Nylon is a polyamide (in that way like a protein). The term polyester also puts our focus on the functional group which is made in the polymerization reaction. Step-growth polymerization involves normal functional group reactions. Polymers result because the monomers have two functional groups per molecule.
Chain-Growth Polymers
Polymers resulting from additions to alkenes are chain-growth polymers. In these processes each addition step results in a longer chain which ends in a reactive site. The mechanism of each addition step is the same, and each addition step adds another monomer to extend the chain by one repeating unit.
Each step in this polymer formation is an addition to an alkene. The mechanism is in most cases a free radical addition. In free radical reactions the pi pair of electrons separates. One of these electrons pairs with an electron from the attacking reagent to form a sigma bond with one of the alkene carbons. and the other electron remains attached to the other alkene carbon. (Curved arrows with only one "barb" on a point are used to follow the path of a single electron in the same way that "double-headed" arrows follow the path of an electron pair.) Intermedates with an unpaired electron are called free radicals, so this step can be described as adding a free radical to an alkene to lengthen the chain by two carbons and generate a new free radical. In its turn this new free radical can add to another molecule of monomer and continue the process.
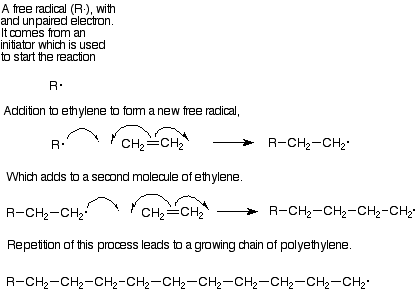
The most important monomers for this process are ethylene (which makes the polymer polyethylene) and substituted ethylenes like vinyl choride (polyvinyl chloride), styrene (phenylethylene, polystyrene), methyl methacrylate (Plexiglas), and acrylonitrile (cyanoethylene, acrylic fibers). Table 15.1, p 427 in Brown lists the structures of these monomers, from which you can deduce the structures of the polylmers.
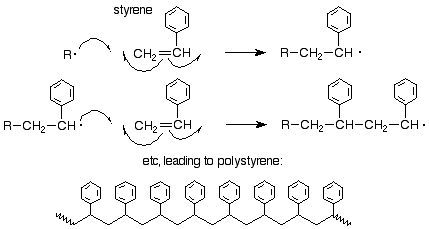
We might ask about the orientation of attack of a radical on a substituted ethylene (vinyl) monomer. It has been shown that the orientation is the same as it is for an electrophilic addition, that is, the free radical attacks the less substituted of the two alkene carbons so as to produce the new free radical at the more substited carbon. Here's an example for styrene:
Other methods of polymerization are also known. The most important is the Ziegler-Natta polymerization which uses a combination of TiCl4 and an alkyl aluminum compound such as Al(CH2CH3)3. This process is discussed in more detail in Sec. 15.5B of Brown.


