5.3: Epoxidation of Unfunctionalized Alkenes
- Page ID
- 168796
Asymmetric epoxidation of unfunctionalized alkenes affords an appealing strategy for the synthesis optically active organic compounds. This section covers some of the recent developments on this protocol.
5.3.1 Manganese-Catalyzed Reactions
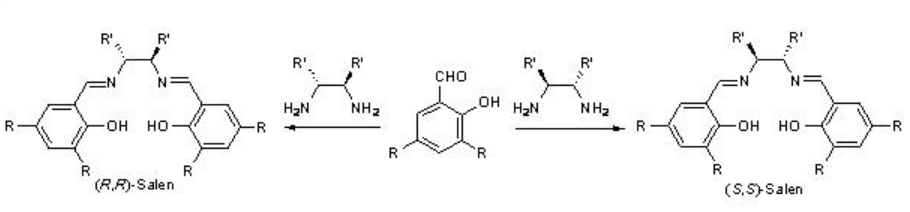
In 1990, Jacoben and Katsuki groups independently reported the chiral Mn-catalzyed asymmetric epoxidation of unfunctionalized alkenes. The catalysts can readily be synthesized by the reaction of Mn(OAc)2 with Schiff base derived from chiral 1,2-diamines and 2-hydroxybenzaldehyde derivatives (Scheme \(\PageIndex{1}\)). Reaction with Mn(OAc)2 in the presence of air gives the Mn(III) complex that may be isolated as the chloro derivative after the addition of lithium chloride.
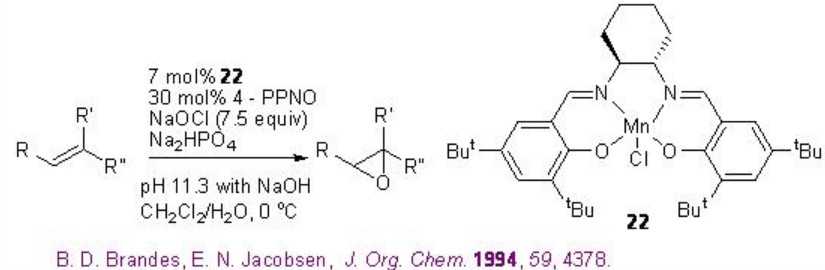
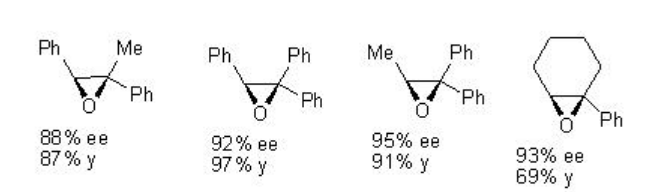
For example, chiral Mn-salen 22 catalyzes the epoxidation of trisubstituted unfunctionalized alkenes with 88-95% ee (Scheme \(\PageIndex{2}\)).
Examples:
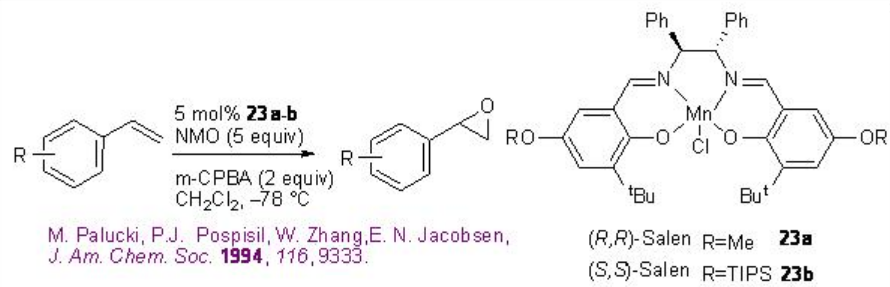
Styrene derivatives can be successfully epoxidized using 23a-b with good enantioselectivity (Scheme \(\PageIndex{3}\)). The reaction is effective using the combination of N -morpholine oxide and m -chloroperbenzoic acid.
Mechanism
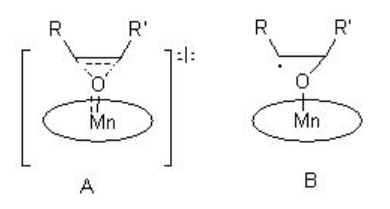
The epoxidation may proceed via a concerted (A) or radical-mediated (B) stepwise manner that depends on the electronic and oxidation state of the oxo species (Scheme \(\PageIndex{4}\)).
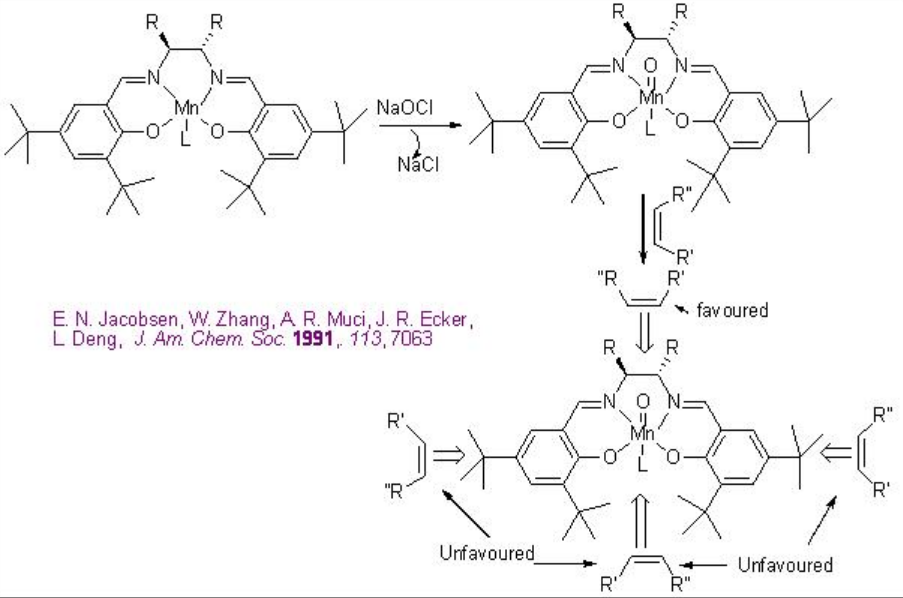
To account the degree and sense of the enantioselectivity, side-on perpendicular approach of the alkene to the high valent metal-oxo intermediate has been invoked (Scheme \(\PageIndex{5}\)).
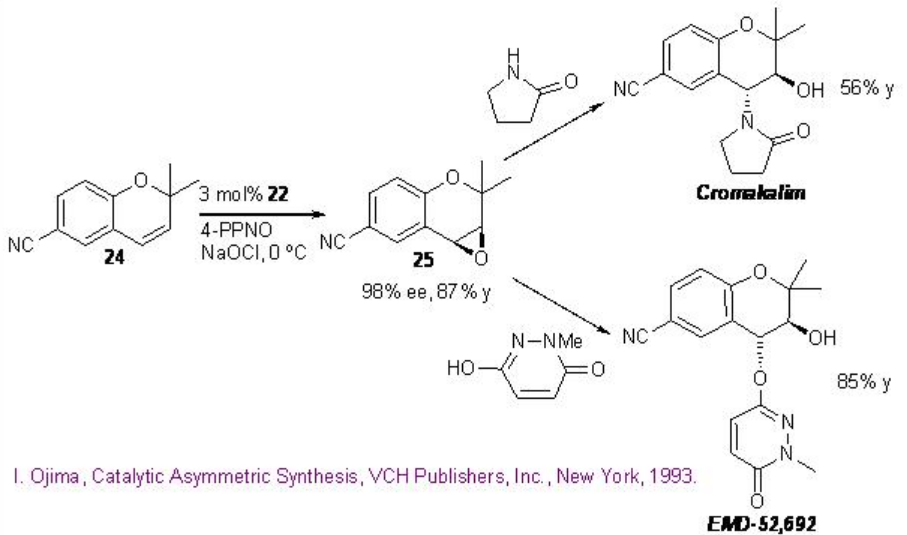
Scheme \(\PageIndex{6}\): Construction of Anti-hypertensive Agents.
Applications
The epoxidation of 6-cyano - 2,2-dimethylchormene 24 with 22 affords 25 that can be converted into anti-hypertensive agents cromakalim and EMD-52692 by reaction with appropriate nitrogen nucleophiles (Scheme \(\PageIndex{6}\)).
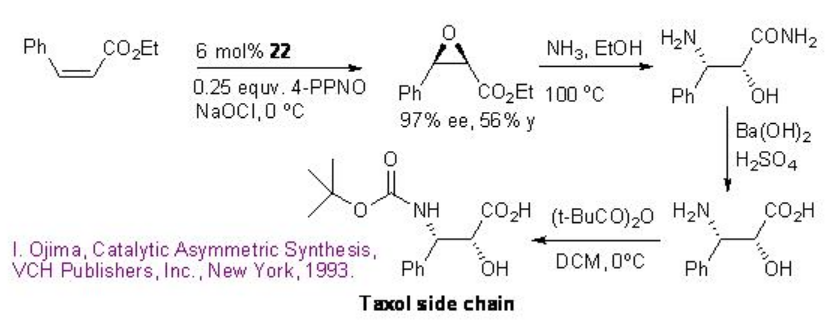
The catalyst 22 has been further utilized for the epoxidation of cis -cinnamic ester in 97% ee and 56% yield that can be converted into taxol side chain by opening of the epoxide with ammonia followed by hydrolysis and protection using (t-BuCO)2O (Scheme \(\PageIndex{7}\)).
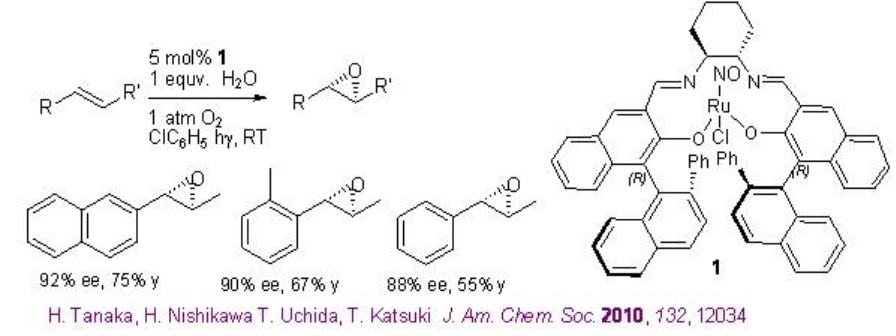
5.3.2 Ruthenium-Catalyzed Aerobic Epoxidation
Chiral Ru(NO)-salen complexes has been found to catalyze the aerobic epoxidation of alkenes in presence of water under visible light irradiation at room temperature (Scheme \(\PageIndex{8}\)). This method is attractive from environmental and economic standpoint. The observed preliminary experimental results suggest that an aqua ligand coordinated with the ruthenium ion acts as a proton transfer agent for the oxygen activation process.
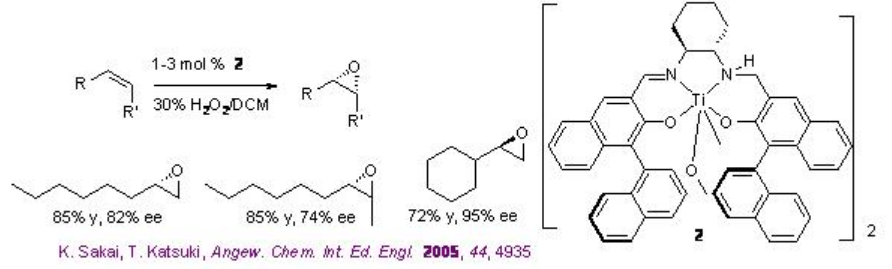
5.3.3 Titanium-Catalyzed Epoxidation with Hydrogen Peroxides
The use of Ti(salan) for the epoxidation of alkenes has been demonstrated in the presence of aqueous H2O2. The reaction is stereospecific and decomposition of H2O2 has not been observed. The most striking feature of this system is aliphatic alkenes that are one of the most challenging substrates for asymmetric epoxidation can be successfully oxidized with high enantioselectivity (Scheme \(\PageIndex{9}\)). Furthermore, the in situ generated titanium complex derived from 3 (SALANEL) and Ti(OiPr)4 in CH2 Cl2 catalyzes the epoxidation of alkenes in the presence of phosphate buffer with excellent enantioselectivity (Scheme \(\PageIndex{10}\)).
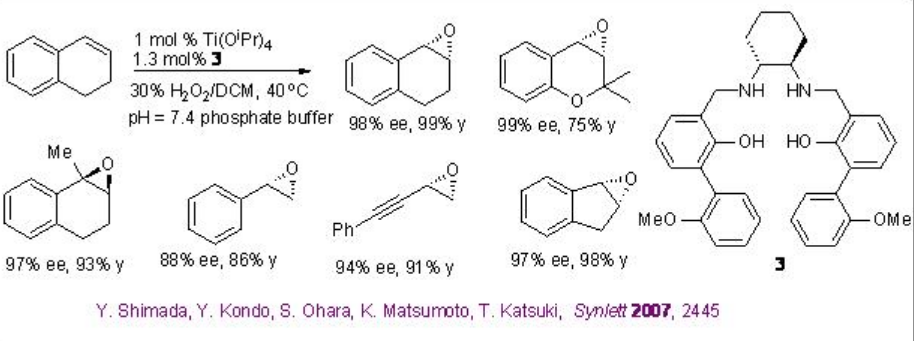
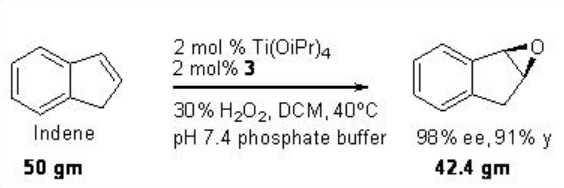
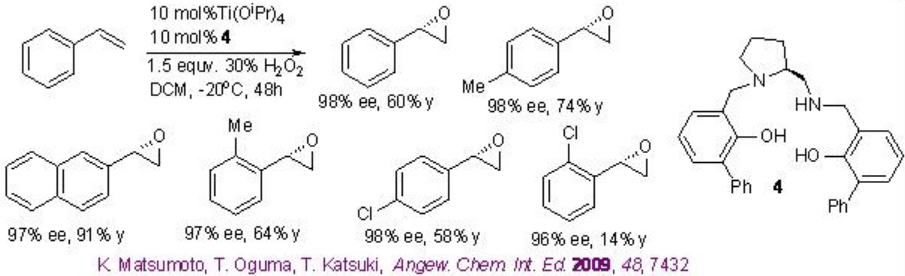
This epoxidation protocol has been successfully applied to a multigram scale synthesis of indene oxide. While the proline-based C1 -symmetric Ti-(salan) from 4 and Ti(OiPr)4 has been found to be excellent catalyst for the epoxidation of styrene derivatives (Scheme \(\PageIndex{11}\)).
5.3.4 Lanthanoid-Catalyzed Epoxidation
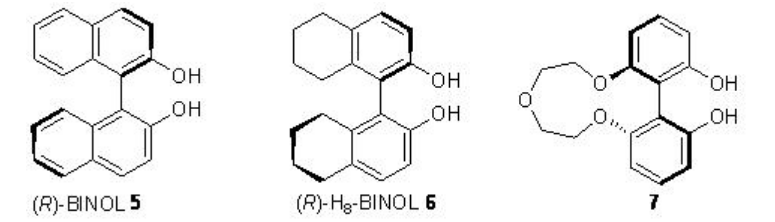
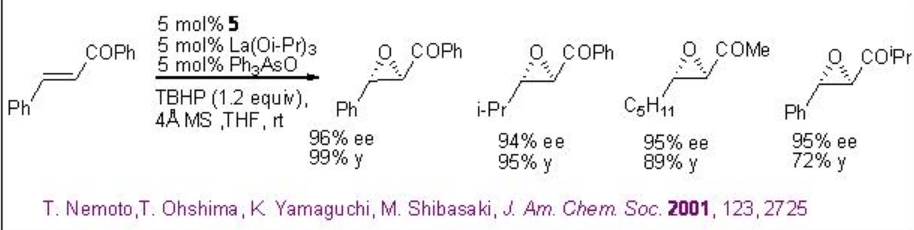
Nucleophilic epoxidation methods represent a viable alternative to electrophilic methods, many of which do not epoxidize electron-poor double bonds. The lanthanide based catalysts derived from chiral ligands 5-7 have been found to be effective in the epoxidation of α,β-unsaturated ketones (Scheme \(\PageIndex{12}\)). It is mainly nucleophilic epoxidation of electron-deficient double bonds through the action of nucleophilic oxidants.
Proposed Mechanism
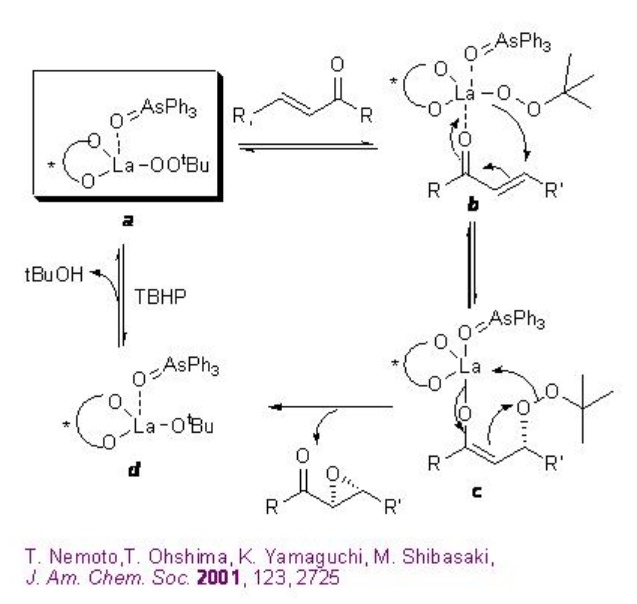
A 1:1:1 mixture of La(OiPr)3 , BINOL and Ph3As=O may afford the active complex a in the reaction medium (Scheme \(\PageIndex{13}\)). Activation of the enone b by coordination to lanthanum metal followed by 1,4-addition of lanthanum peroxide may lead to the formation of enolate c that could provide the epoxide and intermediate d . The latter with TBHP can provide the active complex a to regenerate the catalytic cycle.
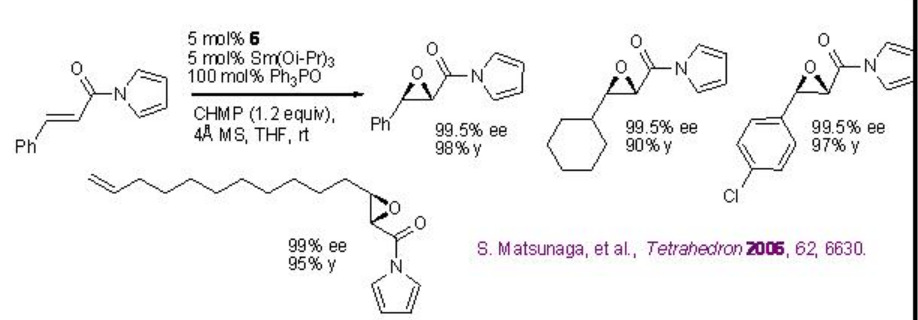
Replacement of La(OiPr)3 by Sm(Oi-Pr)3 , (R)-BINOL 5 by (R)-H8-BINOL 6, Ph3As=O by Ph3P=O and TBHP by CHMP greatly enhances the yield and enantiomeric purity under similar condition for alkenes bearing amides (Scheme \(\PageIndex{14}\)).
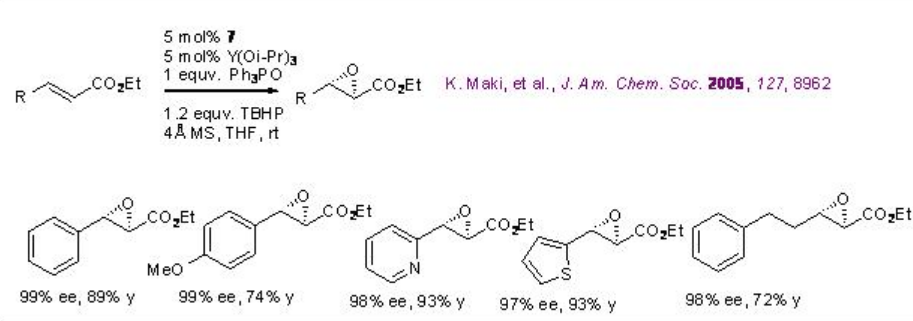
The catalyst derived from 7 and Y(OiPr)3 catalyzes the epoxidation of α,β -unsaturated esters with excellent enantioselectivity (Scheme \(\PageIndex{15}\)). The system is compatible with alkenes bearing heteroaromatic rings.
5.3.5 Organocatalysis
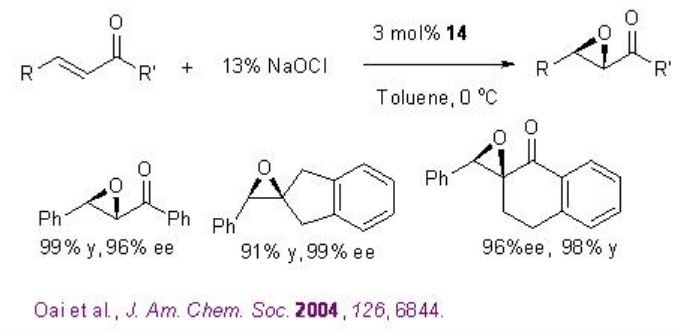
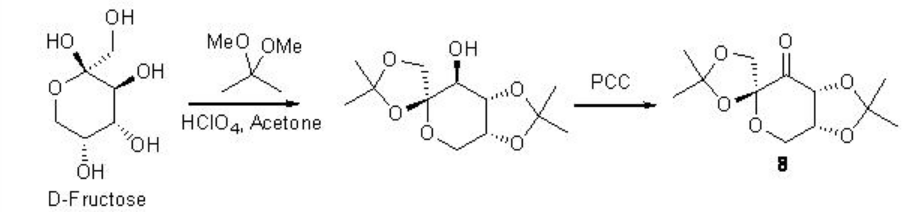
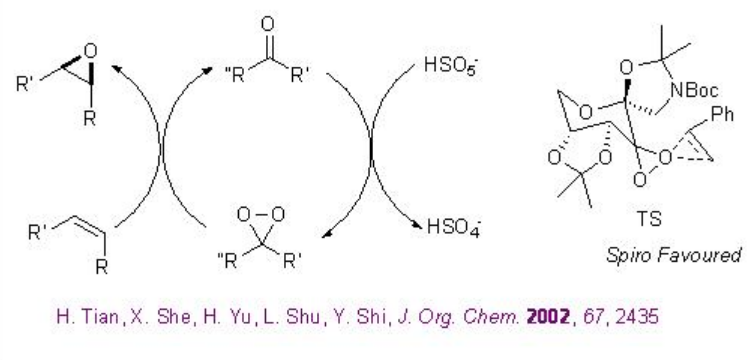
Remarkable progress has been made on the asymmetric epoxidation of alkenes using organo catalysis. Chiral ketones are among the some of the most developed epoxidation catalysts. Active dioxirane is generated from ketone and oxone (potassium peroxomonosulfate) or hydrogen peroxide under milder reaction conditions. Among the many useful chiral ketones reported, fructose derived ketone developed by Shi group is the most reliable catalyst with respect to high enantioselectivity and broad substrate scope (Scheme \(\PageIndex{16}\)).
For example, in presence of 8 (typically 20-30 mol%), a variety of trisubstituted alkenes proceed reaction with excellent enantioselectivity (Scheme \(\PageIndex{17}\)).
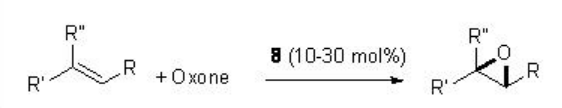
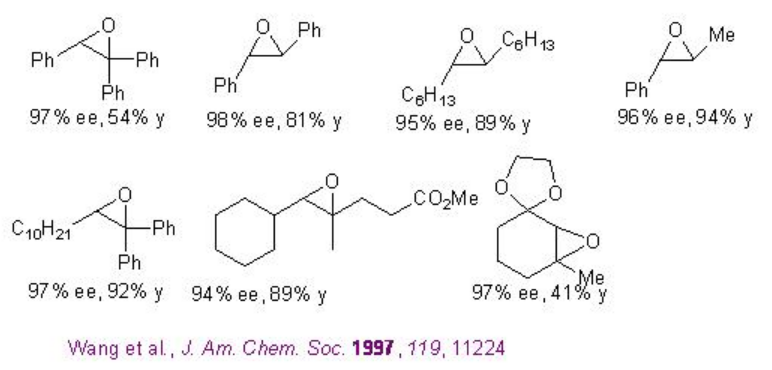
Examples:
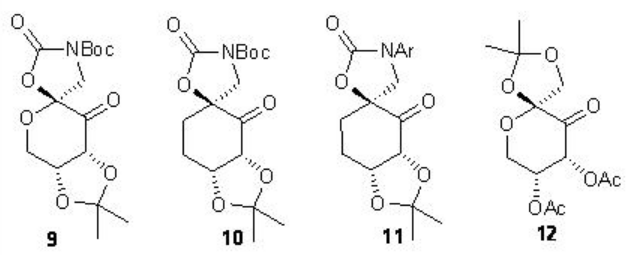
In case of cis and terminal alkenes, the glucose-derived ketone 9 with N -Boc oxazolidinone provides high enantioselectivity. A carbocyclic analogue 10 and N -aryl substituted variants 11 have also been introduced for the epoxidation of styrene derivatives and cis -disubstituted alkenes. Furthermore, the chiral ketone 12 with electron-withdrawing acetate has been found to catalyze the epoxidation of α,β -unsaturated ester with high enantioselectivies.
Proposed Mechanism
Scheme \(\PageIndex{18}\) shows the proposed catalytic cycle and the most favored transition state for the chiral ketone based epoxidations in the presence of oxone as terminal oxidant.
The chiral ketone-catalyzed epoxidation has been subsequently found to be effective using the combination of hydrogen peroxide and acetonitrile as an alternative oxidant. For example, chiral ketone 8 has been used for the epoxidation of a variety of alkenes with comparable yields and enantioselectivity (Scheme \(\PageIndex{19}\)).
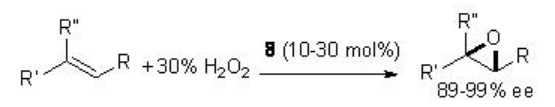
Proposed Mechanism
In this protocol, acetonitrile reacts with hydrogen peroxide to generate peroxyimidic acid and then reacts with the ketone to give the active dioxirane. Under these conditions, a stoichiometric amount of the amide is generated as a product.
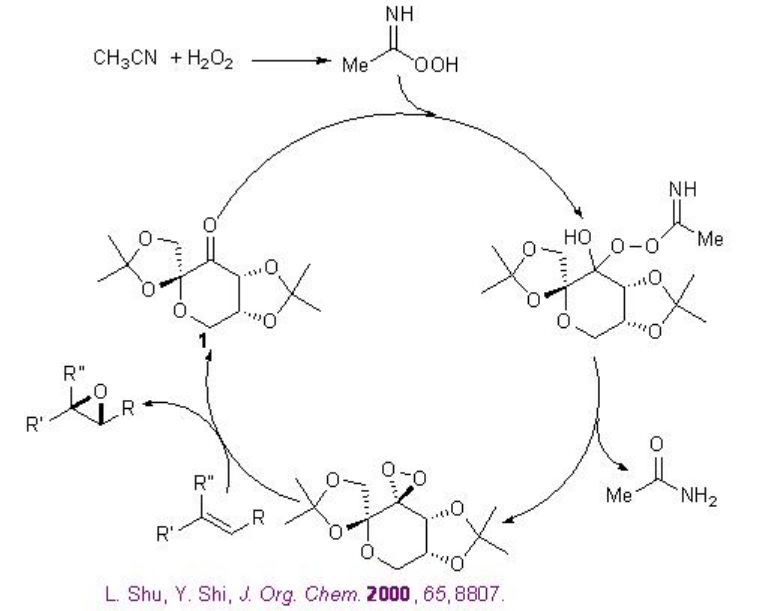
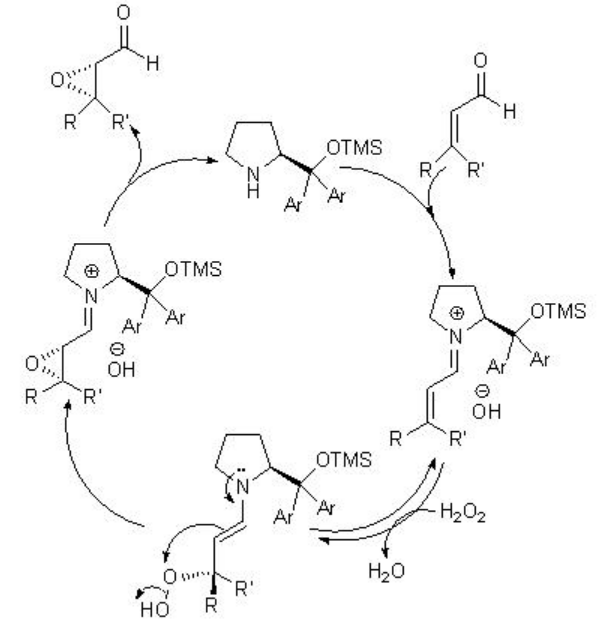
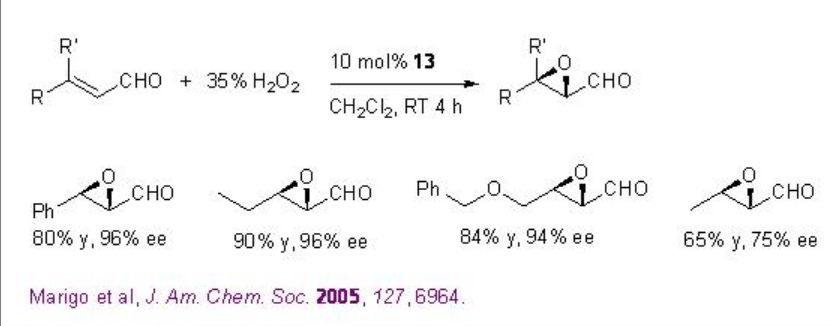
Besides the chiral ketones, chiral amine based catalysts 13 and 14 have been explored for the epoxidation of unfunctionalized alkenes. For example, chiral pyrrolidine 15 has been used for the α,β -unsaturated aldehydes with excellent enantioselectivity in the presence of 35% H2O2 (Scheme \(\PageIndex{20}\)). α,β -Unsaturated aldehydes containing an aromatic substituent at the β -position are good substrates affording the epoxides with high diastereo- and enantioselectivities.
Proposed Mechanism
The proposed mechanism states that the reaction takes place through the Weitz-Scheffer mechanism (Scheme \(\PageIndex{22}\)). The addition of hydrogen peroxide to the β -carbon atom of the electrophilic iminium ion is reversible and the attack on the electrophilic oxygen atom by the nucleophilic enamine determines the product stereochemistry.
While chiral N -spiro ammonium salt 14 bearing an axially chiral binaphthyl unit functions as phase transfer catalyst for the epoxidation of enones with high enantioselectivity (Scheme \(\PageIndex{22}\)). The hydroxyl groups are appropriately bonded to recognize and activate the enone substrate by hydrogen bonding.