2.3: Carbometallation and Carbocyclization Reactions
- Page ID
- 168775
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Organometallic compounds add to carbon-carbon multiple bonds to give a new organometallic species, which could be further modified to yield new carbon-carbon bonds. These processes are called as “carbometallation reactions”. It primarily refers to the relationship between the reactants and products (Scheme \(\PageIndex{1}\)). This section covers some examples of the asymmetric carbometallation reactions using Rh, Cu and Pd-based systems.
Rhodium-Catalyzed Reactions
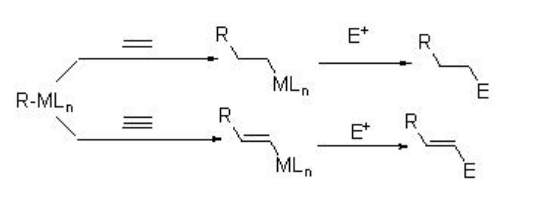
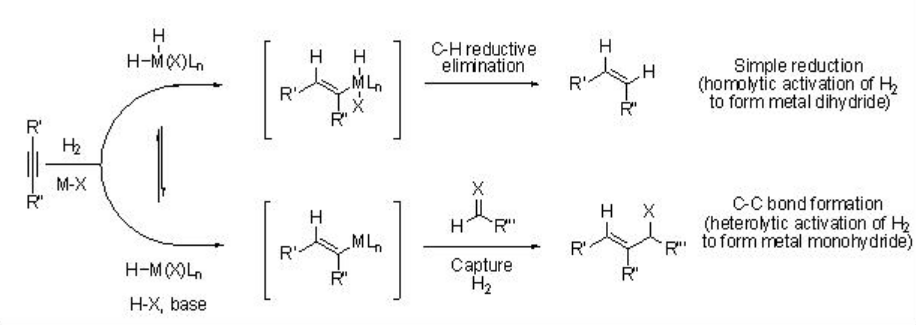
Hydrogen-mediated carbon-carbon bond formation has emerged as powerful industrial process in chemical industries. For example, the hydroformylation and Fischer-Tropsch reactions are well known for the hydrogen-mediated carbon-carbon bond formation reactions. These processes require heterolytic activation of molecular hydrogen to give monohydride species, where the C-H reductive elimination pathway is disabled. Addition of a metal hydride to carbon-carbon multiples bonds (i.e., alkene and alkyne) give organometallic species that could be rapidly captured by an electrophile (i.e., aldehydes and imine) prior to its reaction with molecular hydrogen via oxidative addition or σ-bond metathesis of carbon-metal bond. Scheme \(\PageIndex{2}\) illustrates metal-dihydride route (leading to hydrogenation) and metal-monohydride route (leading to C-C bond formation) with an alkyne.
Formation of the monohydride organometallic species depends on the choice of the catalytic system. For example, the heterolytic activation of molecular hydrogen is observed with cationic rhodium complexes in the presence of base. The reaction takes place via the oxidation addition of the molecular hydrogen with metal species followed by a base induced reductive elimination of HX (Scheme \(\PageIndex{3}\)).
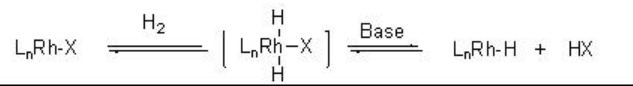
For example, Scheme \(\PageIndex{4}\) presents enantioselective reductive cyclization of 1,6-enynes using Rh(COD)2 OTf and ( R )-BINAP in the presence of molecular hydrogen. This carbocyclization reaction is compatible with various functional groups, however, the yield and enantioselectivity of the product depends on the structure of 1,6-enynes and the ligands.
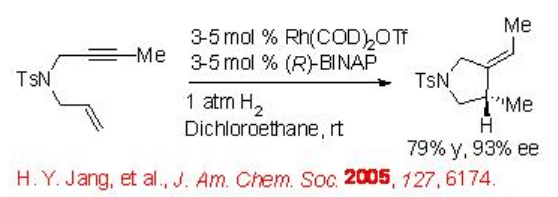
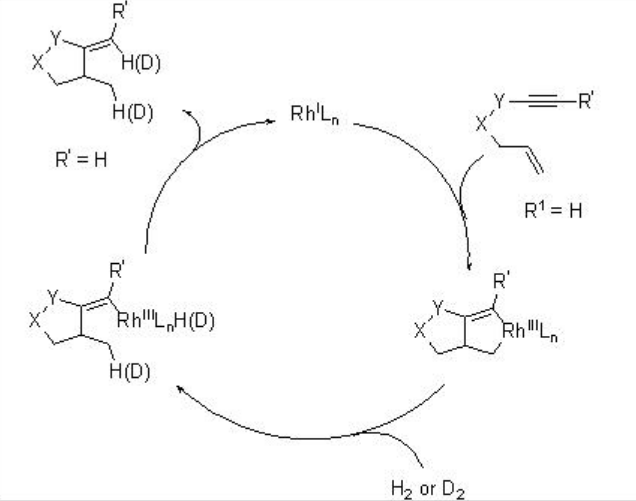
A possible mechanism has been proposed for this reaction based on deuterium labeling control experiments (Scheme \(\PageIndex{5}\)). The catalytic cycle starts with cycloaddition of RhLn and 1,6-enyne forming rhodacyclopentene. Homolytic hydrogen activation via oxidative addition of molecular hydrogen or σ-bond metathesis may lead to the formation of vinyl-rhodium vinyl species that could afford cyclization product by reductive elimination to complete the catalytic cycle.
1,4-Conjugate addition of organometallic reagents to α,β -unsaturated carbonyl compounds afford effective method for carbon-carbon bond formation. Much effort has been on the development of asymmetric version of the reaction using a series of catalytic systems. The first reductive aldol cyclization of keto-enone with phenylboronic acid has been shown utilizing Rh[(COD)Cl]2 and (R)-BINAP with yield and enantioselectivity (Scheme \(\PageIndex{6}\)).
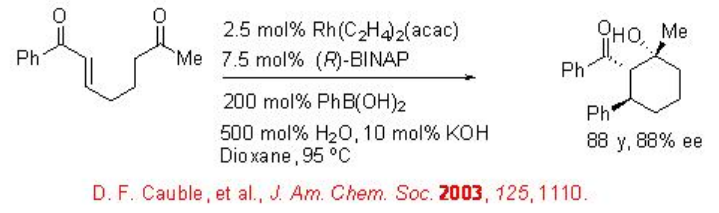
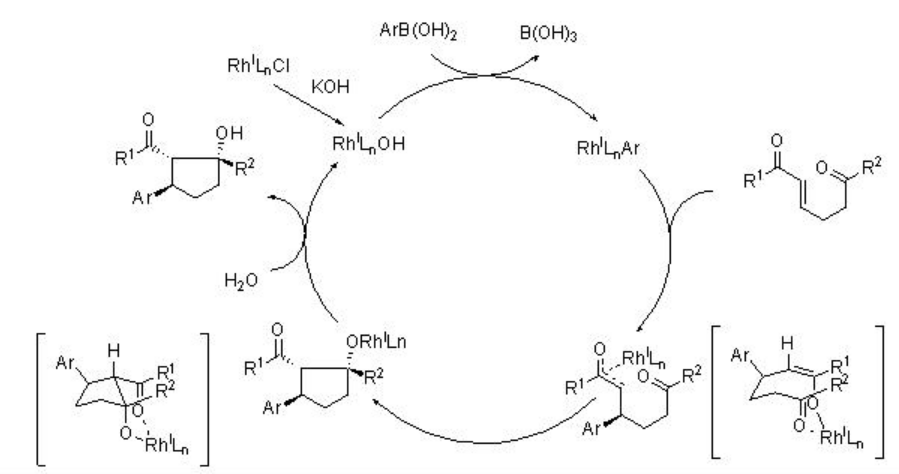
The mechanism of the reaction is presented in Scheme 7. The observed stereochemistry has been rationalized by assuming Z -enolate formation.
Copper-Catalyzed Reactions
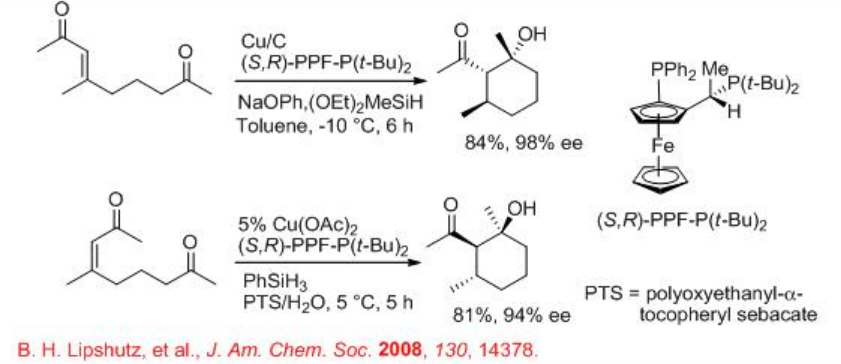
CuH is found to be highly efficient catalyst for the asymmetric reductive aldol cyclization of keto-enones to give the target product as a singly diastereoisomer with high enantiopuritiy (Scheme \(\PageIndex{8}\)). These reactions use ferrocenylphosphines, (S, R)-PPE-P(t -Bu)2, as effective chiral ligands in the presence of silane as a hydride source (Scheme \(\PageIndex{8}\)). These reactions can also be carried out under heterogeneous as well as aqueous conditions with surfactant.
Palladium-Catalyzed Reactions
The palladium-catalyzed cross-coupling reactions of aryl or alkenyl halides with alkenes in the presence of base are among the powerful reactions in organic synthesis to construct carbon-carbon bonds. The asymmetric version of the reaction is also well explored. Scheme \(\PageIndex{9}\)-\(\PageIndex{12}\) illustrates some examples for the intramolecular and intermolecular Heck reactions. In 1970, the Heck reaction was discovered and, in 1989, the first example of asymmetric intramolecular Heck reactions appeared using Pd(OAc)2 with (R)-BINAP with moderate enantioselectivity (Scheme \(\PageIndex{9}\)).
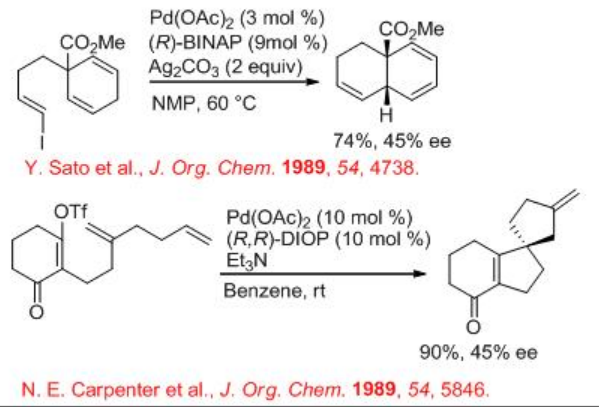
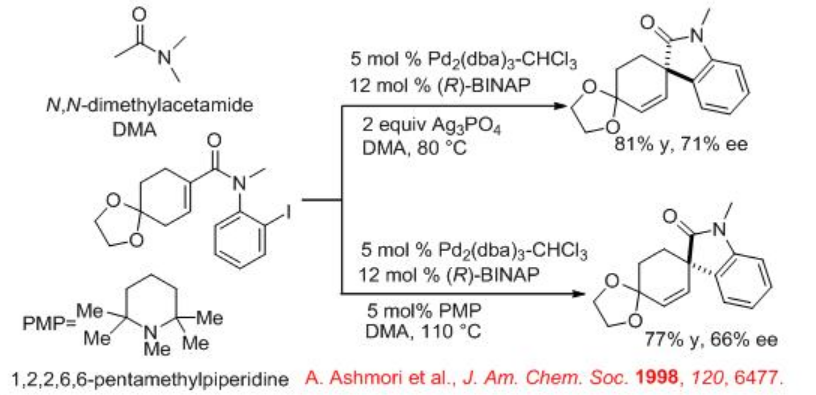
The intramolecular Heck reaction finds wide applications in organic synthesis. Among those applications the synthesis of optically active oxindoles having a quaternary asymmetric center has been considerably explored. Because the oxindole moiety serves as useful synthetic intermediate in the synthesis of numerous natural products. For example, (E)- α,β -unsaturated-2-iodoanilide undergoes cyclization in the presence of Pd2 (dba)3-CHCl3 and (R)-BINAP to give oxindoles with (S) or (R) configuration under cationic and neutral conditions, respectively. It is noteworthy that a dramatic switching in the direction of asymmetric induction has been observed between the two conditions even though the same chiral ligand (R)-BIANP is employed. In these reactions, Ag3PO4and PMP act as HI scavenger.
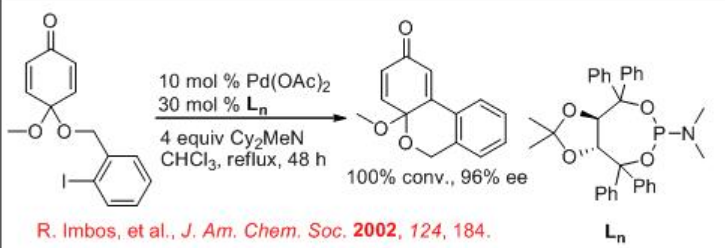
The use of TADDOL-based monophosphoramide has been demonstrated instead of BINAP in the reaction of intramolecular cyclization of cyclohexadienone derivatives (Scheme \(\PageIndex{11}\)). This reaction can be performed in the absence of silver salt.
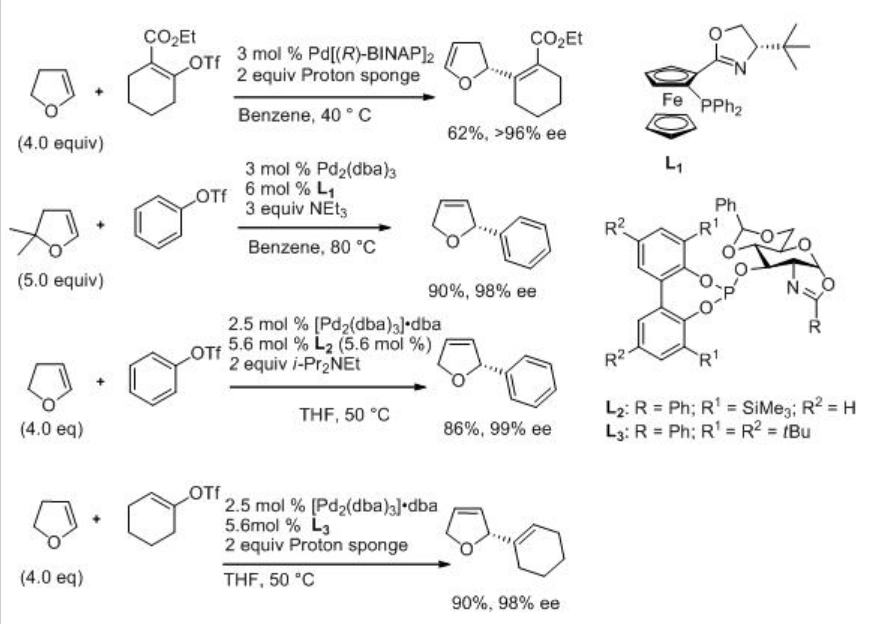
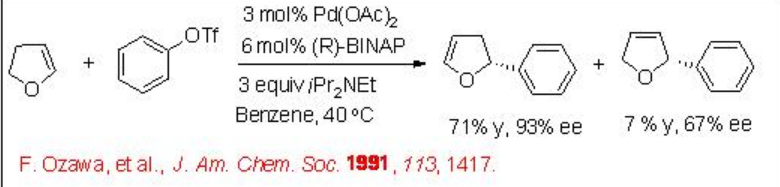
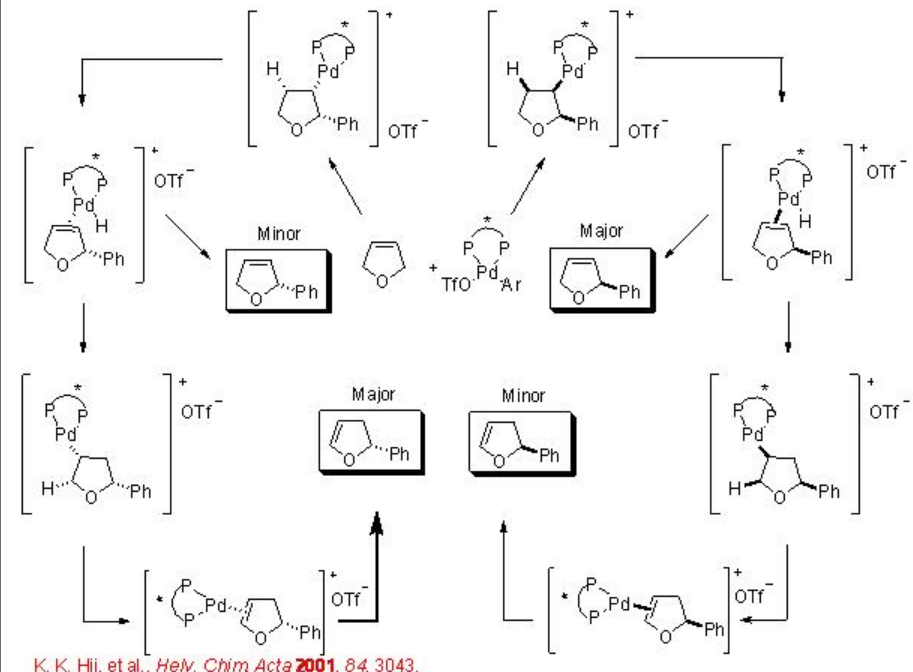
Intermolecular Heck reaction is also well studied. For example, dihydrofuran reacts with phenyl triflate to give 2-phenyl-2,3-dihydrofuran along with small amount 2-phenyl-2,5-dihydrofuran in the presence of Pd-BINAP with excellent enantioselectivity (Scheme \(\PageIndex{12}\)). A mechanism has been proposed to explain the high enantioselectivity of the major product and inversion configuration of the minor product (Scheme \(\PageIndex{13}\)). It involves a kinetic resolution process that enhances the enantioselectivity of the major product.
Scheme \(\PageIndex{14}\) exemplifies the reactions of 2,3-dihydrofuran and 2,2-dimethyl-2,3-dihydrofuran with phenyl triflate, 2-carbethoxy cyclohexenyl triflate and cyclohexenyl trilfate using palladium complexes with oxazoline based aminophosphine and (D-glucosamine)phosphiteoxazoline as the ligands. The reactions are effective affording the products with excellent enantioselectivity.