30.4: Steroids
- Page ID
- 22404
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The term steroid applies to compounds containing hydrogenated cyclopentanophenanthrene carbon skeleton:
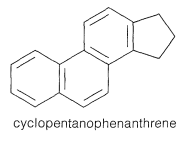
Most steroids are alcohols, and accordingly are named as sterols. Important examples include cholesterol, ergosterol, estradiol, stigmasterol, and other representative sterols given in Table 30-2. As you can see from their structures, most possess the same ring skeleton but vary considerably in their peripheral structural features, stereochemistry, and in the degree of ring unsaturation.
Table 30-2: Representative Steroids
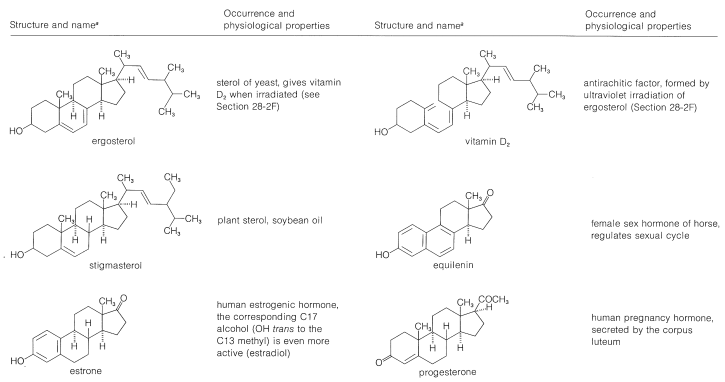
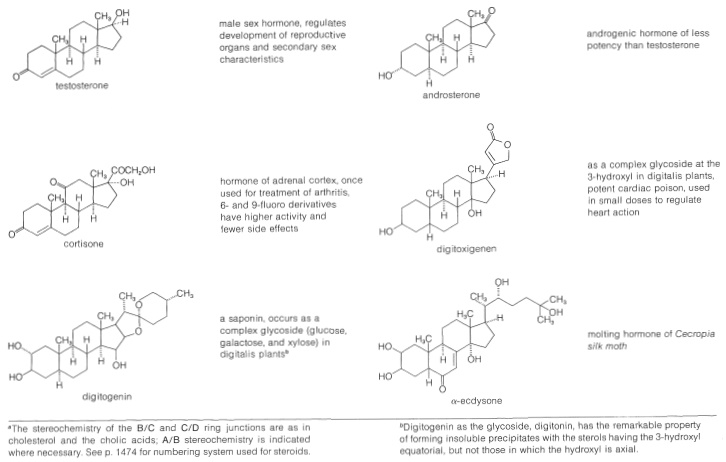
Sterols are widely distributed in both plants and animals. Many are of vital importance to animal physiology, such as cholesterol, the bile acids, vitamin D, sex hormones, and corticoid hormones. Many have value as medicinals, such as the cardiac glycosides, hormones, and steroidal antibiotics. The occurrence and physiological properties of representative steroids are included in Table 30-2.
Cholesterol
Cholesterol is an unsaturated alcohol of formula \(\ce{C_{27}H_{45}OH}\) that has long been known to be the principal constituent of human gall stones and has received notoriety in recent years for its connection with circulatory ailments, particularly hardening of the arteries. Cholesterol, either free or in the form of esters, actually is widely distributed in the body, particularly in nerve and brain tissue, of which it makes up about one sixth of the dry weight. The function of cholesterol in the body is not understood; experiments with labeled cholesterol indicate that cholesterol in nerve and brain tissue is not rapidly equilibrated with cholesterol administered in the diet. Two things are clear: Cholesterol is synthesized in the body and its metabolism is regulated by a highly specific set of enzymes. The high specificity of these enzymes may be judged from the fact that the very closely related plant sterols, such as sitosterol, are not metabolized by the higher animals, even though they have the same stereochemical configuration of all the groups in the ring and differ in structure only near the end of the side chain:
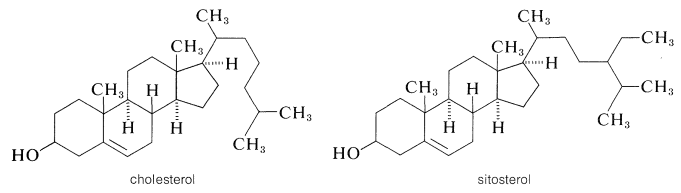
The accepted numbering system for the steroid nucleus and attached side chains is illustrated for cholesterol in \(4\). The methyl groups at the junction of rings \(A\) and \(B\) \(\left( \ce{C_{10}} \right)\) and rings \(C\) and \(D\) \(\left( \ce{C_{13}} \right)\) are called angular methyls. To avoid misinterpretation of structure and stereochemistry, methyl groups and hydrogens at ring junctions should be explicitly written as \(\ce{CH_3}\) or \(\ce{H}\). The stereochemistry is specified by a solid line if the atom or group is above the ring plane \(\left( \beta \right)\), and by a dashed line if below the ring plane \(\left( \alpha \right)\). Thus compound \(5\), 5\(\alpha\)-cholestan-3\(\beta\)-ol, which is obtained by the reduction of cholesterol, implies in the name that the hydroxyl at \(\ce{C_3}\) is above the ring plane and that the hydrogen at \(\ce{C_5}\) is below the ring plane (i.e., the \(A\)/\(B\) rings, have the trans-decalin stereochemistry; Section 12-9).
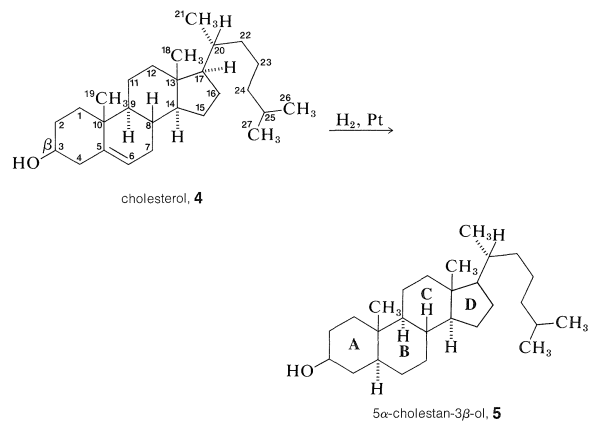
Structure of Cholesterol
Although cholesterol was recognized as an individual chemical substance in 1812, all aspects of its structure and stereochemical configuration were not settled until about 1955. The structural problem was a very difficult one, because most of cholesterol is saturated and not easily degraded. Fortunately, cholesterol is readily available, so that it was possible to use rather elaborate degradative sequences, which would have been quite out of the question with some of the rarer natural products.
The first step in the elucidation of the structure of cholesterol was the determination of the molecular formula, first incorrectly as \(\ce{C_{26}H_{44}O}\) in 1859 and then correctly as \(\ce{C_{27}H_{46}O}\) in 1888. The precision required to distinguish between these two formulas is quite high, because \(\ce{C_{26}H_{44}O}\) has \(83.80\%\) \(\ce{C}\) and \(11.90\%\) \(\ce{H}\), whereas \(\ce{C_{27}H_{46}O}\) has \(83.87\%\) \(\ce{C}\) and \(11.99\%\) \(\ce{H}\). Cholesterol was shown in 1859 to be an alcohol by formation of ester derivatives and in 1868 to possess a double bond by formation of a dibromide. By 1903 the alcohol function was indicated to be secondary by oxidation to a ketone rather than an aldehyde. The presence of the hydroxyl group and double bond when combined with the molecular formula showed the presence of four carbocyclic rings. Further progress was only possible by oxidative degradation.
The structure proof for cholesterol paralleled that for two other important steroids, the so-called bile acids, cholic and desoxycholic acid, which function to help solubilize fats in the intestinal tract. Proof that cholesterol and the bile acids have the same general ring system was achieved by dehydration and reduction of cholesterol to two different hydrocarbons, 5\(\alpha\)-cholestane and 5\(\beta\)-cholestane (coprostane), which differ only in the stereochemistry of the junction between rings \(A\) and \(B\):
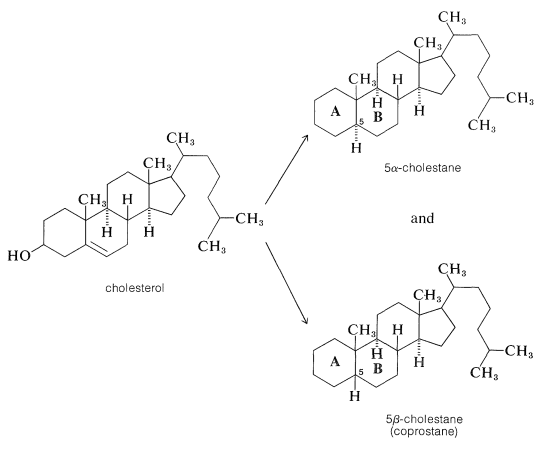
Oxidation of 5\(\beta\)-cholestane, but not 5\(\alpha\)-cholestane, gave an acid that turned out to be identical with cholanic acid obtained by dehydration of cholic acid at \(300^\text{o}\) followed by hydrogenation:
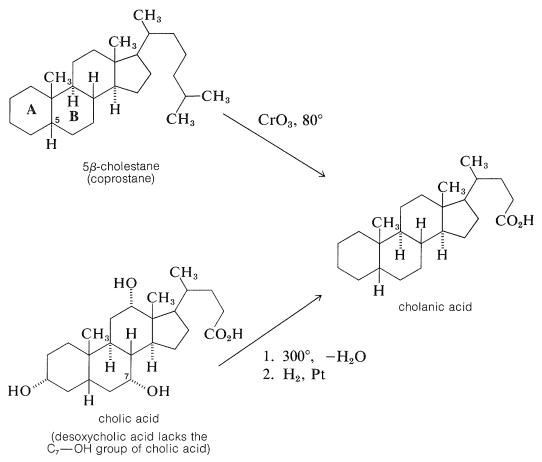
Once the connection between cholesterol and the bile acids was established, further work on the structure proof was directed towards degradation experiments on the bile acids which, with their hydroxyl groups on rings \(B\) and \(C\), offered more possible degradation reactions than cholesterol. Outstanding contributions toward the structure proof were made by the German chemists H. Wieland and A. Windaus, both of whom were honored by the award of the Nobel Prize in chemistry. Wieland received the award in 1927 and Windaus in 1928. Despite their many years of effort, the structure proposed by Windaus in 1928 for desoxycholic acid was only tentative and was unspecific as to the location of two carbons.
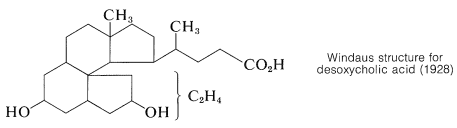
Serious doubt as to the correctness of the Windaus structure came as a result of an x-ray study of ergosterol by J. D. Bernal in 1932. He pointed out that the x-ray evidence indicated ergosterol to be a long, rather flat molecule. Steroids with the ring system corresponding to the Windaus structure would have a globular shape. This observation stimulated further work and a re-examination of the evidence that eventually led to the correct structure. The details of this research may be found elsewhere.\(^3\)
Synthesis of Steroids
One of the most notable achievements of the 1950's was the total synthesis of a number of important steroids, including estrone, cholesterol, cortisone, androsterone, and testosterone. The courses of some of these syntheses are extraordinarily complex and involve large numbers of steps. Although they have not surpassed Nature in providing practical quantities of synthetic steroids, they have led to the development of key reactions of general use in organic synthesis. An especially useful reaction for building fused ring systems is the so-called Robinson annelation reaction developed by Sir Robert Robinson (Nobel Prize, 1947) and J. W. Cornforth (Nobel Prize, 1975). The reaction involves a Michael addition to an \(\alpha\),\(\beta\)-unsaturated ketone immediately followed by an aldol addition:
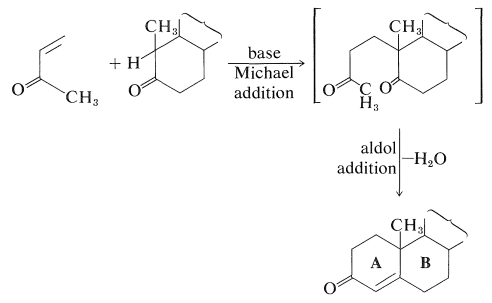
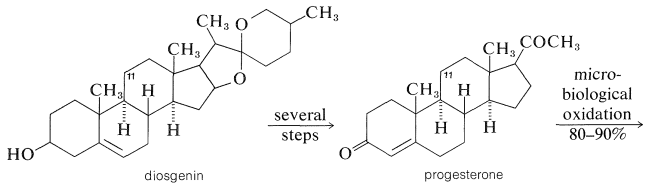
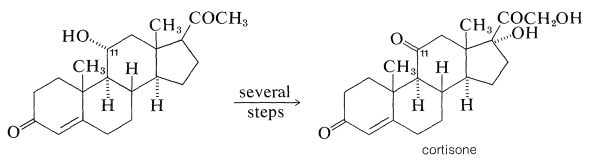
The pharmacological importance of many natural steroids has stimulated much synthetic work in an effort to obtain practical quantities of naturally occurring and unnatural steroids. Oftentimes a combination of biosynthesis and organic synthesis works best. For example, the need for large quantities of cortisone derivatives for therapeutic use in treatment of arthritis and similar metabolic diseases has led to intensive research on synthetic approaches for methods of producing steroids with oxygen functions at \(\ce{C_{11}}\), which is not a particularly common point of substitution in steroids.
An efficient way of doing this is by microbiological oxidation. Cortisone can be manufactured on a relatively large scale from the saponin, diosgenin, which is isolated from tubers of a Mexican yam of the genus Dioscorea. Diosgenin is converted to progesterone, then by a high-yield (\(80\%\)-\(90\%\)) oxidation with the mold, Rhizopus nigricans, to 11-hydroxyprogesterone and finally to cortisone:
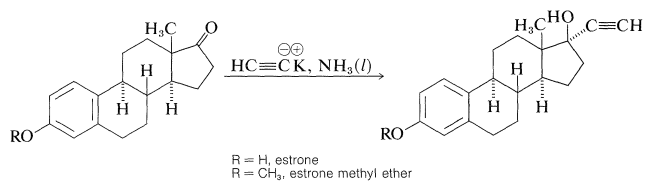
Especially important steroid derivatives in use today are the synthetic estrogens, 17-\(\alpha\)-ethynylestradiol and its 3-\(\ce{OCH_3}\) derivative, mestranol:
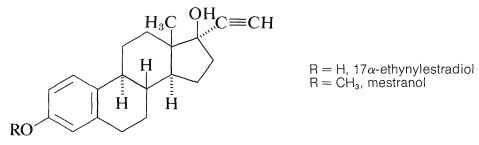
Both of these compounds have potent estrogenic activity (inhibit ovulation) and are widely used as oral contraceptives. They are synthesize from the natural estrogen, estrone, by the following reaction:
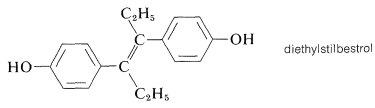
A compound known as diethylstilbestrol (DES) also exhibits estrogenic activity even though it is unrelated structurally to steroidal estrogens. It has acquired notoriety as a possible cause of uterine cancer. Diethylstilbestrol has been used extensively as an additive in cattle and chicken feed because it gives a greater gain in weight for a given amount of feed.
\(^3\)See, for example, the excellent and authoritative account give by L. F. Fieser and M. Fieser, Steroids, Van Nostrand Reinhold Co., New York, 1959, Chapter 3.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


