23.14: Carcinogenic Nitrogen Compounds
- Page ID
- 22349
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Amines
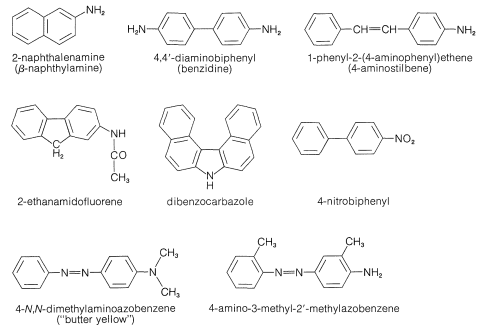
Everyone who works with organic chemicals should be aware that a number of arenamines are carcinogens. The most dangerous examples (see Figure 23-8) are known to induce human bladder cancer. These chemicals were used widely in the chemical industry (mostly in azo dye manufacture) long before they were recognized as hazardous carcinogens. Voluntary action and appropriate legislation now controls the industrial uses of these substances, and there also are some controls for uses in research and teaching. It is important to be aware of the potential hazards of known carcinogens and to recognize that all chemicals, both organic and inorganic, should be treated with great respect if their thermodynamic and physiological properties are not known. Carcinogenic character is just one of many possible hazards.
Azo Compounds
Other nitrogen compounds besides amines are known to be carcinogenic. For example, certain azo dyes (see Figure 23-8) have been found to produce tumors in animals. This fact has caused concern for human health because, as we indicated in Section 23-10C, azo dyes are coloring agents that are used in many products. They certainly are not all carcinogenic, but the structural requirements for a compound to show this property may change completely the toxic properties of a chemical. For example, the \(\ce{N}\),\(\ce{N}\)-diethylamino analog of "butter yellow" (Figure 23-8) is apparently hazardous.
\(\ce{N}\)-Nitroso Compounds
We have seen that \(\ce{N}\)-nitroso compounds are formed from secondary amines and nitrous acid:
\[\ce{R_2NH} + \ce{HONO} \overset{\ce{H}^\oplus}{\longrightarrow} \ce{R_2N-NO} + \ce{H_2O}\]
\(\ce{N}\)-nitroso compounds also can be formed from carboxamides and nitrous acid:

Some of these nitrosoamines and nitrosoamides are known to be potent carcinogens for some animals, which is reason to suspect they also may be carcinogenic for humans. However, it is clear that there may be very marked differences in carcinogenic properties of a given compound for different animal species.
Why some of these substances have carcinogenic activity is a matter of chemical interest. Recall from Section 23-10 that nitrosation of amines usually leads to cleavage of a \(\ce{C-N}\) bond in the sense \(\ce{C} \vdots \colon \ce{N}\). The carbon fragment ultimately is transferred to some nucleophilic atom. In effect, this means that nitrosamines can function as alkylating agents and, in a biological system, the functions that probably would be alkylated are the nucleophilic sites along the polymeric protein or nucleic acid chains. It is not difficult to appreciate that alkylation of these substances may well disrupt the pattern of normal cell growth.
There is an unresolved problem related to the carcinogenic properties of nitroso compounds. You probably are aware (if you read the labels on food packages) that sodium nitrite is added to many packaged meat products. Sodium nitrite prevents the growth of harmful bacteria, thereby retarding spoilage, and it also enhances the appearance by maintaining the red look of fresh meat. There is a possibility that nitrite may have adverse effects on human health by nitrosating the amino and amide functions of proteins in the presence of acids. This possibility has to be balanced against the alternative threat to human health if the use of nitrite were discontinued, that of increased food spoilage. In any case, it seems clear that the amount of sodium nitrite actually used in most processing is in excess of that needed to retard bacterial decay.
There are many other chemicals that are active alkylating agents besides nitrosamines, and some are unquestionably carcinogenic (see Figure 23-9), whereas others apparently are not. In fact, it is a paradox that some of the most useful synthetic drugs in treating certain forms of cancer are alkylating agents. Several of these are shown in Figure 23-10. They all have two or more active centers in the molecule that enable them to form cross-links between protein or nucleic acid molecules.
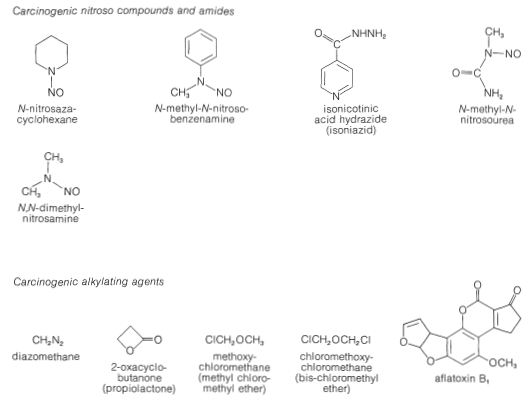

It should be recognized that not all of the carcinogenic substances loosed on mankind are the result of modern technology. The most potent carcinogens known, which are lethal in test animals at levels of a few parts per billion, are mold metabolites called aflatoxins. These substances are complex non-nitrogenous, heterocyclic oxygen compounds, which often are formed by molds growing on cereal grains, peanuts, and so on. (See Figure 23-9)
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."


