13.10: Protecting Groups in Organic Synthesis
- Page ID
- 22056
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the major problems in organic synthesis is the suppression of unwanted side reactions. Frequently the desired reaction is accompanied by reaction at other parts of the molecule, especially when more than one functional group is present. Functional groups usually are the most reactive sites in the molecule, and it may be difficult or even impossible to insulate one functional group from a reaction occurring at another. Therefore any proposed synthesis must be evaluated at each step for possible side reactions that may degrade or otherwise modify the structure in an undesired way. To do this will require an understanding of how variations in structure affect chemical reactivity. Such understanding is acquired through experience and knowledge of reaction mechanism and reaction stereochemistry.
To illustrate the purpose and practice of protecting groups in organic synthesis, let us suppose that the synthesis of cis-2-octene, which we outlined in Section 13-7, has to be adapted for the synthesis of 5-octyn-1-ol. We could write the following:
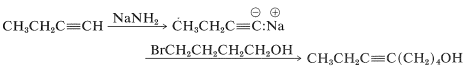

However, the synthesis as written would fail because the alkyne is a weaker acid than the alcohol (Section 11-8), and the alkynide anion would react much more rapidly with the acidic proton of the alcohol than it would displace bromide ion from carbon:
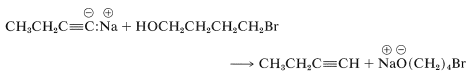

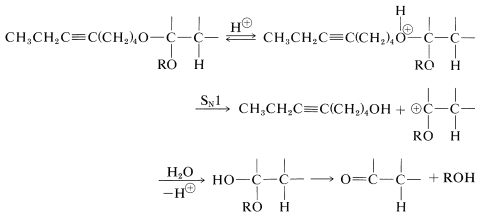
The hydroxyl group of 4-bromo-1-butanol therefore must be protected before it is allowed to react with the alkynide salt. There are a number of ways to protect hydroxyl groups, but one method, which is simple and effective, relies on the fact that unsaturated ethers of the type 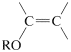
 are very reactive in electrophilic addition reactions (Section 10-4). An alcohol readily adds to the double bond of such an ether in the presence of an acid catalyst:
are very reactive in electrophilic addition reactions (Section 10-4). An alcohol readily adds to the double bond of such an ether in the presence of an acid catalyst:
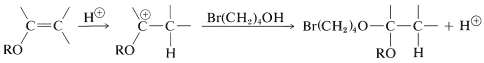

The protected compound is a much weaker acid than the alkyne, and the displacement reaction can be carried out with the alkynide salt without difficulty. To obtain the final product, the protecting group must be removed, and this can be done in dilute aqueous acid solution by an \(S_\text{N}1\) type of substitution (Sections 8-7D and 8-7E):

Some Common Protecting Groups in Organic Synthesis
Hydroxyl \(\left( \ce{OH} \right)\) protecting groups in Organic Synthesis
Protection of alcohols:
Acetyl \(\left( \ce{Ac} \right)\) – Removed by acid or base.
Benzoyl \(\left( \ce{Bz} \right)\) – Removed by acid or base, more stable than \(\ce{Ac}\) group.
Benzyl (\(\ce{Bn}\), \(\ce{Bnl}\)) – Removed by hydrogenolysis. \(\ce{Bn}\) group is widely used in sugar and nucleoside chemistry.
\(\beta\)-Methoxyethoxymethyl ether (MEM) – Removed by acid.
Dimethoxytrityl, [bis-(4-methoxyphenyl)phenylmethyl] (DMT) – Removed by weak acid. DMT group is widely used for protection of 5′-hydroxy group in nucleosides, particularly in oligonucleotide synthesis.
Methoxymethyl ether (MOM) – Removed by acid.
Methoxytrityl [(4-methoxyphenyl)diphenylmethyl, MMT) – Removed by acid and hydrogenolysis.
p-Methoxybenzyl ether (PMB) – Removed by acid, hydrogenolysis, or oxidation.
Methylthiomethyl ether – Removed by acid.
Pivaloyl \(\left( \ce{Piv} \right)\) – Removed by acid, base or reductant agents. It is substantially more stable than other acyl protecting groups.
Tetrahydropyranyl (THP) – Removed by acid.
Trityl (triphenylmethyl, \(\ce{Tr}\)) – Removed by acid and hydrogenolysis.
Silyl ether (most popular ones include trimethylsilyl (TMS), tert-butyldimethylsilyl (TBDMS), tri-iso-propylsilyloxymethyl (TOM), and triisopropylsilyl (TIPS) ethers) – Removed by acid or fluoride ion. (such as \(\ce{NaF}\), TBAF (Tetra-n-butylammonium fluoride, HF-Py, or HF-NEt3)). TBDMS and TOM groups are used for protection of 2′-hydroxy function in nucleosides, particularly in oligonucleotide synthesis.
Methyl Ethers – Cleavage is by TMSI in DCM or MeCN or Chloroform. An alternative method to cleave methyl ethers is BBr3 in DCM
Ethoxyethyl ethers (EE) – Cleavage more trivial than simple ethers e.g. 1N Hydrochloric acid
Amine protecting groups in Organic Synthesis
Protection of amines:
Carbobenzyloxy (Cbz) group – Removed by hydrogenolysis
p-Methoxybenzyl carbonyl (Moz or MeOZ) group – Removed by hydrogenolysis, more labile than Cbz
tert-Butyloxycarbonyl (BOC) group (Common in solid phase peptide synthesis) – Removed by concentrated, strong acid. (such as HCl or CF3COOH)
9-Fluorenylmethyloxycarbonyl (FMOC) group (Common in solid phase peptide synthesis) – Removed by base, such as piperidine
Acetyl (Ac) group is common in oligonucleotide synthesis for protection of N4 in cytosine and N6 in adenine nucleic bases and is removed by treatment with a base, most often, with aqueous or gaseous ammonia or methylamine. Ac is too stable to be readily removed from aliphatic amides.
Benzoyl (Bz) group is common in oligonucleotide synthesis for protection of N4 in cytosine and N6 in adenine nucleic bases and is removed by treatment with a base, most often with aqueous or gaseous ammonia or methylamine. Bz is too stable to be readily removed from aliphatic amides.
Benzyl (Bn) group – Removed by hydrogenolysis
Carbamate group – Removed by acid and mild heating.
p-Methoxybenzyl (PMB) – Removed by hydrogenolysis, more labile than Benzyl
3,4-Dimethoxybenzyl (DMPM) – Removed by hydrogenolysis, more labile than p-methoxybenzyl
p-methoxyphenyl (PMP) group – Removed by Ammonium cerium(IV) nitrate (CAN)
Tosyl (Ts) group – Removed by concentrated acid (HBr, H2SO4) & strong reducing agents (sodium in liquid ammonia or sodium naphthalenide)
Other Sulfonamides (Nosyl & Nps) groups – Removed by samarium iodide, tributyltin hydride
Carbonyl protecting groups in Organic Synthesis
Protection of carbonyl groups:
Acetals and Ketals – Removed by acid. Normally, the cleavage of acyclic acetals is easier than of cyclic acetals.
Acylals – Removed by Lewis acids.
Dithianes – Removed by metal salts or oxidizing agents.
Carboxylic acid protecting groups in Organic Synthesis
Protection of carboxylic acids:
Methyl esters – Removed by acid or base.
Benzyl esters – Removed by hydrogenolysis.
tert-Butyl esters – Removed by acid, base and some reductants.
Silyl esters – Removed by acid, base and organometallic reagents.
Orthoesters – Removed by mild aqueous acid to form ester, which is removed according to ester properties.
Oxazoline – Removed by strong hot acid (pH < 1, T > 100 °C) or alkali (pH > 12, T > 100 °C), but not e.g. LiAlH4, organolithium reagents or Grignard (organomagnesium) reagents
Phosphate protecting groups in Organic Synthesis
2-cyanoethyl – removed by mild base. The group is widely used in oligonucleotide synthesis.
Methyl (Me) – removed by strong nucleophiles e.c. thiophenole/TEA.
Terminal alkyne protecting groups in Organic Synthesis
propargyl alcohols in the Favorskii reaction,
silyl groups, especially in protection of the acetylene itself
Orthogonal protection in Organic Synthesis
Orthogonal protection is a strategy allowing the deprotection of multiple protective groups one at a time each with a dedicated set of reaction conditions without affecting the other. It was introduced in the field of peptide synthesis by Robert Bruce Merrifield in 1977. As a proof of concept orthogonal deprotection is demonstrated in a photochemical transesterification by trimethylsilyldiazomethane utilizing the kinetic isotope effect:
Due to this effect the quantum yield for deprotection of the right-side ester group is reduced and it stays intact. Significantly by placing the deuterium atoms next to the left-side ester group or by changing the wavelength to 254 nm the other monoarene is obtained.
Contributors and Attributions
John D. Robert and Marjorie C. Caserio (1977) Basic Principles of Organic Chemistry, second edition. W. A. Benjamin, Inc. , Menlo Park, CA. ISBN 0-8053-8329-8. This content is copyrighted under the following conditions, "You are granted permission for individual, educational, research and non-commercial reproduction, distribution, display and performance of this work in any format."



