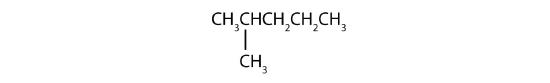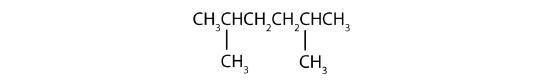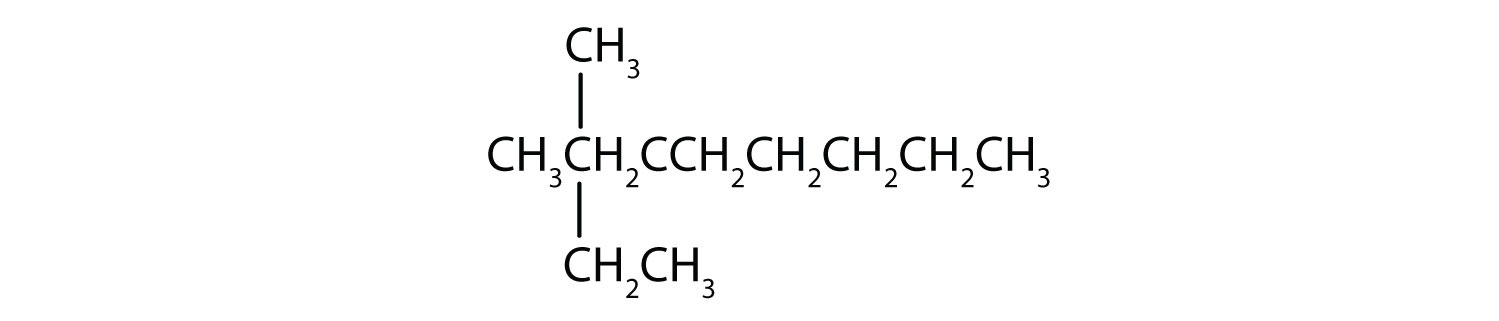12.6: Naming Alkanes
- Page ID
- 86263
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- To name alkanes by the IUPAC system and write formulas for alkanes given IUPAC names
As noted in previously, the number of isomers increases rapidly as the number of carbon atoms increases: there are 3 pentanes, 5 hexanes, 9 heptanes, and 18 octanes, etc. It would be difficult to assign each compound unique individual names that we could remember easily. A systematic way of naming hydrocarbons and other organic compounds has been devised by the International Union of Pure and Applied Chemistry (IUPAC). These rules, used worldwide, are known as the IUPAC System of Nomenclature. (Some of the names mentioned earlier, such as isobutane, isopentane, and neopentane, do not follow these rules and are called common names.)
In the IUPAC system, a compound is named according to the number of carbons in the longest continuous chain (LCC) or parent chain and the family it belongs to. Atoms or groups attached to this carbon chain, called substituents, are then named, with their positions indicated by a numerical prefix at the beginning of the name:
Prefix (substituent) – Parent (# carbons) – Suffix (family name)
2-methylpropane
(Table \(\PageIndex{1}\)) below lists the IUPAC parent names that are used for charbon chains containing 1 to 10 carbons, along with straight-chain alkane examples for each. Notice that the suffix for each example in this table is -ane, which indicates these are members of the alkane family.
| Number of Carbons | Parent Chain (LCC) Name | Example Alkane Name | Example Condensed Structural Formula |
|---|---|---|---|
| 1 | meth- | methane | CH4 |
| 2 | eth- | ethane | CH3CH3 |
| 3 | prop- | propane | CH3CH2CH3 |
| 4 | but- | butane | CH3CH2CH2CH3 |
| 5 | pent- | pentane | CH3CH2CH2CH2CH3 |
| 6 | hex- | hexane | CH3CH2CH2CH2CH2CH3 |
| 7 | hept- | heptane | CH3CH2CH2CH2CH2CH2CH3 |
| 8 | oct- | octane | CH3CH2CH2CH2CH2CH2CH2CH3 |
| 9 | non- | nonane | CH3CH2CH2CH2CH2CH2CH2CH2CH3 |
| 10 | dec- | decane | CH3CH2CH2CH2CH2CH2CH2CH2CH2CH3 |
Atoms or groups of atoms that branch off the parent chain are called substituents. When the substituent is a carbon or group of carbons, such as –CH3 or –CH2CH3, it is called an alkyl group. Alkyl groups are alkanes that have had one hydrogen removed to allow for binding to a main chain carbon and are named by replacing the -ane suffix of the parent hydrocarbon with -yl. For example, the –CH3 group derived from methane (CH4) results from subtracting one hydrogen atom and is called a methyl group. Removing a hydrogen from ethane, CH3CH3, gives –CH2CH3, or the ethyl group. The alkyl groups we will use most frequently are listed in Table \(\PageIndex{2}\). Alkyl groups are not independent molecules; they are parts of molecules that we consider as a unit to name compounds systematically.
| Parent Alkane | Alkyl Group | Condensed Structural Formula | ||
|---|---|---|---|---|
| methane | 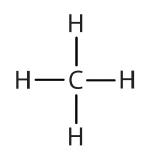 |
methyl | 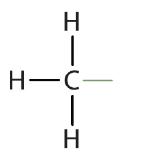 |
CH3– |
| ethane | 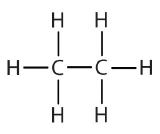 |
ethyl | 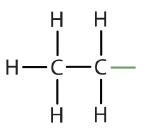 |
CH3CH2– |
| propane | 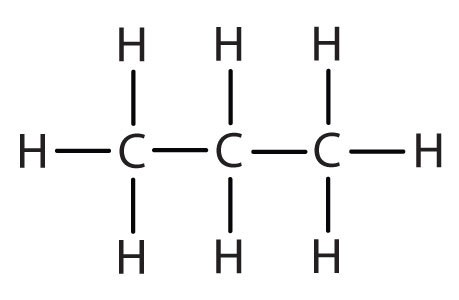 |
propyl | 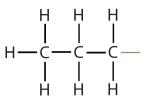 |
CH3CH2CH2– |
| isopropyl | 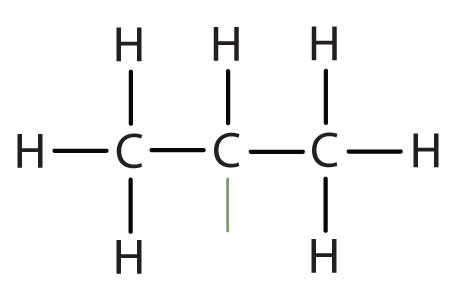 |
(CH3)2CH– | ||
| butane | 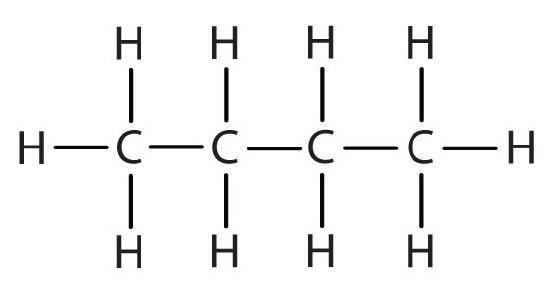 |
butyl | 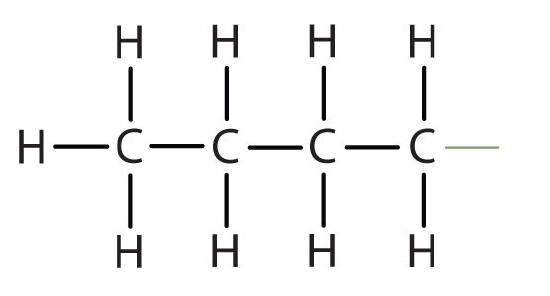 |
CH3CH2CH2CH2– |
| sec-butyl | ||||
| isobutyl | ||||
| tert-butyl (tBu) | ||||
Simplified IUPAC rules for naming alkanes are as follows (demonstrated in Example \(\PageIndex{1}\)).
Step 1: Name the parent chain. Find the longest continuous chain, (it may not always be the most obvious chain written in one line), and name according to the number of carbon atoms it contains. Add the suffix -ane to indicate that the molecule is an alkane. Use Table \(\PageIndex{1}\) as a reference to start, but it is a good idea to commit these to memory.
Step 2: Number the carbon atoms in the parent chain, giving carbons with any substituents attached the lowest number possible. These numbers are used to locate where substituents are attached to a main chain.
Step 3: Name any substituents (including the location number). If the same alkyl group appears more than once, the numbers of all the carbon atoms to which it is attached are expressed. If the same group appears more than once on the same carbon atom, the number of that carbon atom is repeated as many times as the group appears. Moreover, the number of identical groups is indicated by the Greek prefixes di-, tri-, tetra-, and so on. These prefixes are not considered in determining the alphabetical order of the substituents. For example, ethyl is listed before dimethyl; the di- is simply ignored. The last alkyl group named is prefixed to the name of the parent alkane to form one word.
Step 4: Write the name of the compound as a single word placing the substituent groups first (in alphabetical order), then the parent name, then the family name. Hyphens are used to separate numbers from the names of substituents; commas separate numbers from each other.
When these rules are followed, every unique compound receives its own exclusive name. The rules enable us to not only name a compound from a given structure but also draw a structure from a given name. The best way to learn how to use the IUPAC system is to practice it, not just memorize the rules. It’s easier than it looks.
Name each compound.
Solution
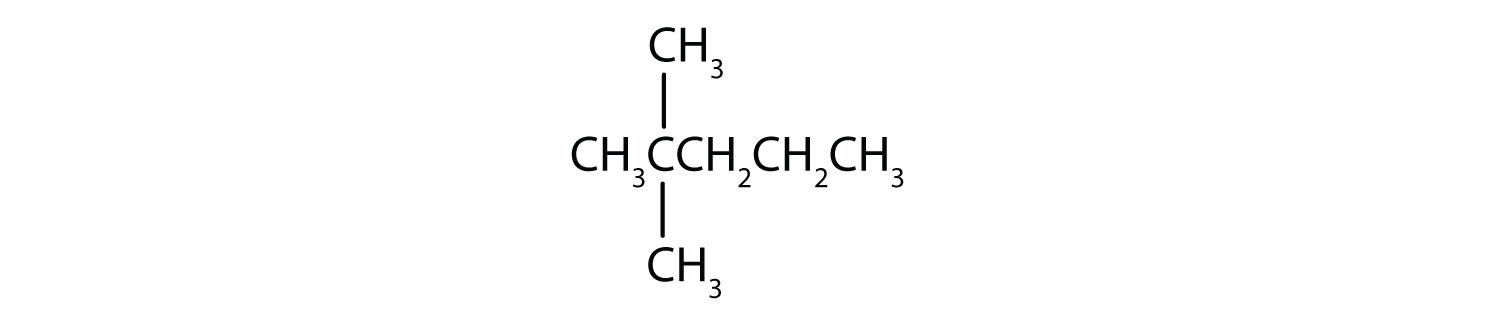
- Step 1: The LCC has five carbon atoms, and so the parent compound name is pentane. Step 2: Number the carbons in the LCC from left to right. Step 3: There is a methyl group attached to carbon #2 of the pentane chain. Step 4: The name is 2-methylpentane.
- Step 1: The LCC has six carbon atoms, so the parent compound is hexane. Step 2: Number the carbons in the LCC from left to right (or right to left, either way will be identical numbering). Step 3: There are two methyl groups attached to the second and fifth carbon atoms. Step 4: The name is 2,5-dimethylhexane.
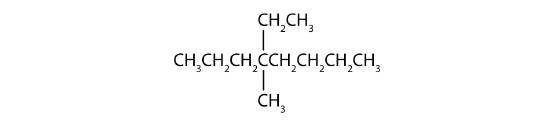
- Step 1: The LCC has eight carbon atoms, so the parent compound is octane. Step 2: Number the carbons in the LCC from left to right to give the lower number. Step 3: There are methyl and ethyl groups, both attached to the fourth carbon atom. Step 4: The correct name is thus 4-ethyl-4-methyloctane.
Name each compound.
Draw the structure for each compound.
- 2,3-dimethylbutane
- 4-ethyl-2-methylheptane
Solution
In drawing structures, always start with the parent chain.
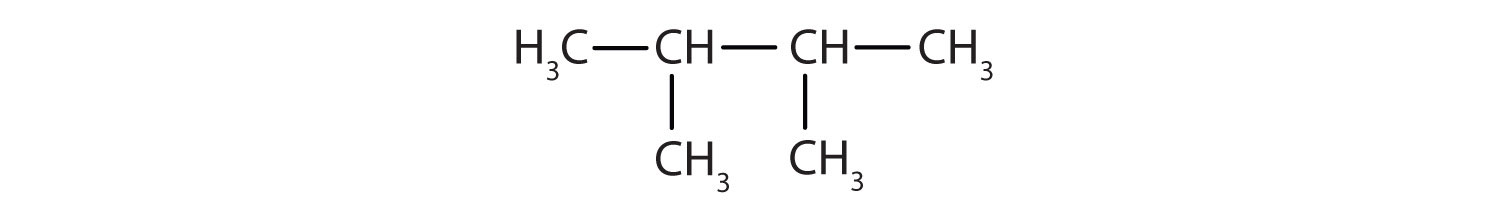
- The parent chain is butane, indicating four carbon atoms in the LCC.
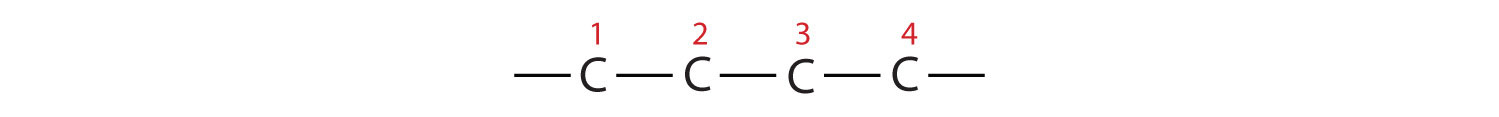
Then add the substituents at their proper positions. You can number the parent chain from either direction as long as you are consistent; just don’t change directions before the structure is done. The name indicates two methyl (–CH3) groups, one on the second carbon atom and one on the third.

Finally, fill in all the hydrogen atoms, keeping in mind that each carbon atom must have four bonds total.
- Adding the substituents at their proper positions gives
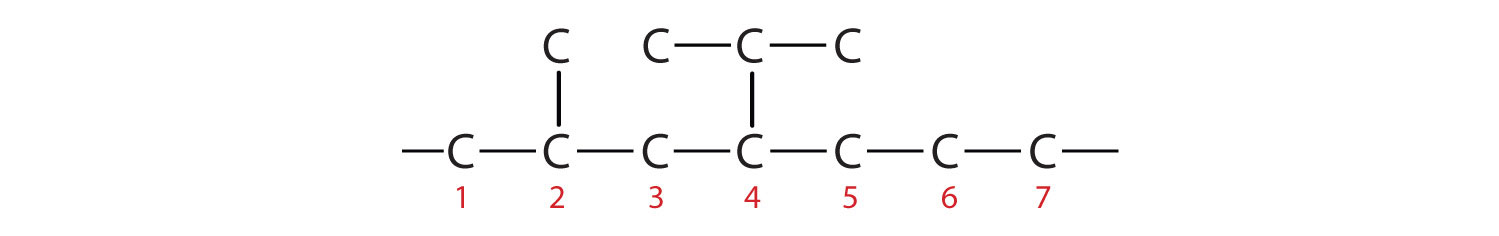
Filling in all the hydrogen atoms gives the following condensed structural formulas (both are correct):
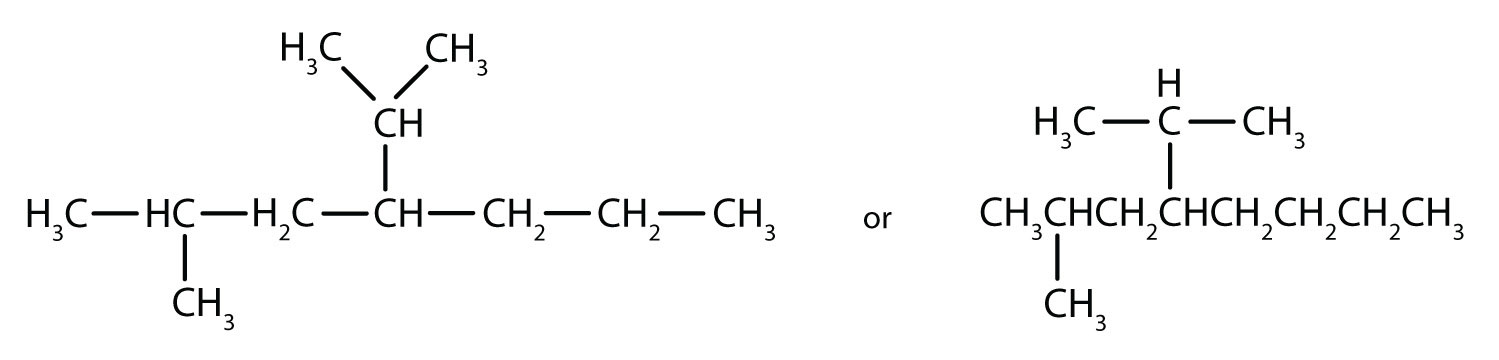
Note that the bonds (dashes) can be shown or not; sometimes they are needed for spacing.
Draw the structure for each compound.
- 4-ethyloctane
- 3-ethyl-2-methylpentane
- 3,3,5-trimethylheptane