5.1: Electromagnetic Spectrum
- Page ID
- 52953
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What are waves?
Waves can come in many sizes. Here we see a large wave crashing on the beach. Other waves can be very small and regular. We normally think of waves as being made of water, but there are forms of energy that take on the characteristics of waves. The idea of a wave has played a major role in our understanding of how the atom is put together and why it behaves the way it does.
Properties of Light
The nuclear atomic model proposed by Rutherford was a great improvement over previous models, but was still not complete. It did not fully explain the location and behavior of the electrons in the vast space outside of the nucleus. In fact, it was well known that oppositely charged particles attract one another. Rutherford's model did not explain why the electrons don't simply move toward, and eventually collide with, the nucleus. Experiments in the early twentieth century began to focus on the absorption and emission of light by matter. These studies showed how certain phenomena associated with light reveal insight into the nature of matter, energy, and atomic structure.
Wave Nature of Light
In order to begin to understand the nature of the electron, we first need to look at the properties of light. Prior to 1900, scientists thought light behaved solely as a wave. As we will see later, this began to change as new experiments demonstrated that light also has some of the characteristics of a particle. First, we will examine the wavelike properties of light.
Visible light is one type of electromagnetic radiation, which is a form of energy that exhibits wavelike behavior as it moves through space. Other types of electromagnetic radiation include gamma rays, x-rays, ultraviolet light, infrared light, microwaves, and radio waves. The figure below shows the electromagnetic spectrum, which is all forms of electromagnetic radiation. Notice that visible light makes up only a very, very small portion of the entire electromagnetic spectrum. All electromagnetic radiation moves through a vacuum at a constant speed of \(2.998 \times 10^8 \: \text{m/s}\). While the presence of air molecules slows the speed of light by a very small amount, we will use the value of \(3.00 \times 10^8 \: \text{m/s}\) as the speed of light in air.
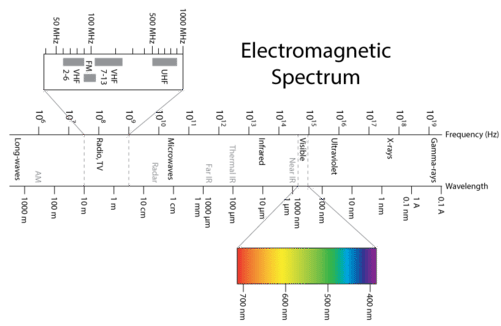
Source: CK-12 Foundation; License: CC BY-NC 3.0(opens in new window))
The figure above shows how the electromagnetic spectrum displays a wide variation in wavelength and frequency. Radio waves have wavelengths of as long as hundreds of meters, while the wavelength of gamma rays are on the order of \(10^{-12} \: \text{m}\). The corresponding frequencies range from \(10^6\) to \(10^{21} \: \text{Hz}\). Visible light can be split into colors with the use of a prism (see below), yielding the visible spectrum of light. Red light has the longest wavelength and lowest frequency, while violet light has the shortest wavelength and highest frequency. Visible light wavelength ranges from about \(400\) to \(700 \: \text{nm}\) with frequencies in the range of \(10^{14} \: \text{Hz}\).
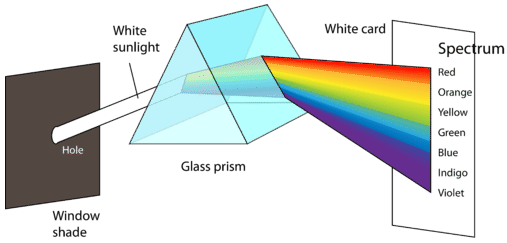
If we could see other wavelengths of energy, would they appear as colors? Use this simulation to explore the electromagnetic spectrum.
Summary
- Electromagnetic radiation is a form of energy.
- Visible light has wavelengths from \(400\)-\(700 \: \text{nm}\).
- The speed of light in air is \(3.00 \times 10^8 \: \text{m/s}\).
Review
- What did Rutherford’s nuclear atomic model not explain?
- Prior to 1900, what did scientists believe about the nature of light?
- What is visible light?
- What is the range of wavelengths for visible light?

