7.2: Amino acids
- Page ID
- 425774
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Define and distinguish amino acids, \(\alpha\)-amino acids, and proteinogenic amino acids.
- Draw Fisher projections and assign D/L or R/S stereodescriptors to proteinogenic amino acids.
- Understand the classification of proteinogenic amino acids based on the characteristics of the side chain.
- Define isoelectric point and understand the ionization states of amino acids under physiological conditions.
- Define essential amino acids and their sources.
What are amino acids?
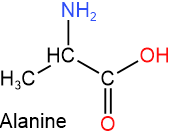 Amino acids are the building blocks of proteins, i.e., they are the monomers of proteins. Amino acids are organic compounds that contain both an amine (\(\ce{-NH2}\)) and a carboxylic acid (\(\ce{-COOH}\)) group in the same molecule. For example, alanine, shown on the right, is an amino acid. Proteins contain a subclass of amino acids called
Amino acids are the building blocks of proteins, i.e., they are the monomers of proteins. Amino acids are organic compounds that contain both an amine (\(\ce{-NH2}\)) and a carboxylic acid (\(\ce{-COOH}\)) group in the same molecule. For example, alanine, shown on the right, is an amino acid. Proteins contain a subclass of amino acids called
an amine (\(\ce{-NH2}\)) on the \(\alpha\ce{C}\) to \(\ce{-COOH}\) group, i.e., both the amine (\(\ce{-NH2}\)) group and the carboxylic acid (\(\ce{-COOH}\)) group are attached to the same \(\ce{C}\). For example, alanine is an
Proteinogenic amino acids are a subclass of \(\alpha\)-amino acids incorporated into proteins during biosynthesis. Twenty proteinogenic amino acids are usually present in proteins, and two additional are included in exceptional cases. This chapter's word "amino acid" refers to the 20 standard proteinogenic amino acids.
Configuration of \(\alpha\ce{C}\) of an
the \(\alpha\ce{C}\) of \(\alpha\)-amino acid have four different groups attached to it: amine (\(\ce{-NH2}\)), carboxylic acid ((\(\ce{-COOH}\)), hydrogen (\(\ce{-H}\)), and alkyl side chain (\(\ce{-R}\), as shown in Figure \(\PageIndex{1}\). There is one exception "glycine" that has two \(\ce{H's}\) at the \(\alpha\ce{C}\)
 Fisher projection
Fisher projection Model
ModelThree groups, i.e., \(\ce{-NH2}\), (\(\ce{-COOH}\), and \(\ce{-H}\) are present in all , and the fourth group, i.e., the side chain (\(\ce{-R}\)) varies in different
The \(\ce{-COOH}\) is acidic (pKa 2-5) and the \(\ce{-NH2}\) is basic (pKa ~10). The \(\ce{-COOH}\) loses its proton and exists as a carboxylate ion ( \(\ce{-COO^{-}}\); the \(\ce{-NH2}\) gains a proton and exists as ammonium ion ( \(\ce{-NH3^{+}}\) at physiological pH of 7.4. These groups ionize the same way if present in the side chain in addition to the \(\alpha\ce{C}\).
Compounds that have a positive charge on one atom and a negative charge on another atom in the same molecule are called zwitterions. Most of the Figure \(\PageIndex{1}\).
A \(\ce{C}\) with four different groups is a chiral center. \(\alpha\ce{C}\) of \(\alpha\ce{C}\)"glycine" that has two \(\ce{H's}\) at the \(\alpha\ce{C}\).presented in Fisher projections with the \(\ce{C}\)-chain placed vertically and the \(\ce{-NH2}\) and \(\ce{-H}\) bonds shown horizontally, as shown in Figure \(\PageIndex{1}\).
The

Proteinogenic amino acids are L-amino acids, with the exception of glycine, which is not chiral. In the R/S system, proteinogenic amino acids are (S) at the \(\alpha\ce{C}\), with the exception of "cysteine" being (R) and glycine not chiral.
The Figure \(\PageIndex{2}\) roteinogenic amino acids are classified based on the nature of the side chain.

Classification of proteinogenic amino acids
\(\alpha\)-Amino acids are classified based on the hydrophobicity of their side chain.
Hydrophobic means "water fearing", i.e., molecules or entities that tend to repel water, not dissolve, or not wetted by water. Hydrophilic means "water-loving", i.e., molecules or entities that tend to mix with, dissolve, or be wetted by water.
Hydrocarbons and other non-polar compounds are hydrophobic compounds that do not dissolve in water. Polar or ionic compounds are usually soluble in water. Based on these criteria, amino acids are classified into the following classes, shown in Figure 7.2.2.
- Nonpolar amino acids with an aliphatic side chain are hydrophobic. Methionine having a thioether group in the side chain is non-polar and placed in this group.
- Nonpolar amino acids with an aromatic side chain are hydrophobic. Tyrosine has phenol as part of its side chain, but it is hydrophobic and placed in this group.
- Polar neutral amino acids with a polar neutral side chain are hydrophilic. This class of side chain contains alcohol (\(\ce{-OH}\)) or amide \(\ce{-CONH2}\) groups in their side chain that hydrogen bond with water but do not ionize.
- Amino acids in a special class include cysteine, glycine, and proline.
- Speciality of cysteine is that its thiol {\(ce{-SH}\) group is easily oxidized forming is a disulfide (\(\ce{S-S}\) bond, which is the only covalent bond in protein besides the amide bonds. Cystein is classified as nonpolar and hydrophobic because {\(ce{-SH}\) is a nonpolar group.
- Glycine is the only proteinogenic acid that has no chiral center. The \(\ce{-H}\) side chain places it at a borderline between hydrophilic and hydrophobic categories, it is considered neutral.
- Proline is the only amino acid with a secondary \(\alpha\)-amine group. The side chain is a five member ring with \(\ce{N}\) of \(\alpha\)-amine as part of the ring. It is classified as a hydrophilic. The ring structure puts restrictions on the allowed configurations when it is incorporated in proteins. It creates a bent or kink in the protein backbone structure.
- Acidic amino acids with an acidic side chain are hydrophilic. These amino acids has carboxylic acid (\(\ce{-COOH}\)) group in their side chain that ionize to anion \(\ce{-COO^{-}}\) under physiological conditions.
- Basic amino acids with a basic side chain are hydrophilic. These amino acids have basic primary or secondary amine groups in their side chain that ionize to cation \(\ce{-NH3^{+}}\) or \(\ce{-NRH2^{+}}\). Histidine has an amine group that has pKa 6.04 and is not ionized at pH 7.4, but it is placed in this group as it ionizes at pH below 6.0.
Acid-base nature of \(\alpha\)-amino acids
The pKa is a measure of the strength of an acid, i.e., the lower the pKa stronger the acid. Amino acids have \(\ce{-COOH}\) group that is acidic with pKa 2-3 and \(\ce{-NH2}\) on adjacent \(\ce{C}\) that is basic with pKa ~40. Conjugate acid of \(\ce{-NH2}\), i.e., \(\ce{-NH3^{+}}\) has pKa ~10. Acids lose their proton when they are in a medium with a pH higher than the pKa of the acid. For example \(\ce{-COOH}\) exist as \(\ce{-COO^{-}}\) in physiological medium with pH ~7.4. Bases gain protons in a medium with a pH lower than the pKa of their conjugate acid. For example, \(\ce{-NH2}\) exist as \(\ce{-NH3^{+}}\) in physiological medium with pH ~7.4. Amino acids exist as zwitterions, i.e., have both cation \(\ce{-NH3^{+}}\) and anion \(\ce{-COO^{-}}\) in the same molecule. Some amino acids have an additional acid or base group in their side chain that also ionizes depending on the pH of the medium.
The gain or loss of protons is an equilibrium process. In a strongly acidic medium, basic groups gain more protons than the protons lost by their acid groups. So, the amino acids have an overall positive charge in a strong acid medium. In a strongly basic medium basic groups gain fewer protons than those lost by their acid groups. So, the amino acids have an overall negative charge in a strongly basic medium. At a certain pH in the middle, an amino acid has an equal positive and negative charge and is neutral overall.
An amino acid's isoelectric point (pI) is the pH at which it has equal positive and negative charges and carries no net charge, i.e., it is neutral overall.
The pKa value of \(\ce{-COOH}\) and \(\ce{-NH3^{+}}\) on the \(\alpha\)-\(\ce{C}\), pKa values of acidic or basic groups in the side chain, and pI values of 20 \(\alpha\)-amino acids found in proteins are listed in Table 1 below.
| Amino acid | Three letters abbreviations | One letter abbreviations | pKa of \(\alpha\)\(\ce{-COOH}\) | pKa of \(\alpha\)\(\ce{-NH3^{+}}\) | pKa of side chain group | Isoelectric point (pI) |
|---|---|---|---|---|---|---|
| Alanine | Ala | A | 2.34 | 9.69 | – | 6.00 |
| Arginine | Arg | R | 2.17 | 9.04 | 12.48 | 10.76 |
| Asparagine | Asn | N | 2.02 | 8.80 | – | 5.41 |
| Aspartic acid | Asp | D | 1.88 | 9.60 | 3.65 | 2.77 |
| Cysteine | Cys | C | 1.96 | 10.28 | 8.18 | 5.07 |
| Glutamic acid | Glu | E | 2.19 | 9.67 | 4.25 | 3.22 |
| Glutamine | Gln | Q | 2.17 | 9.13 | – | 5.65 |
| Glycine | Gly | G | 2.34 | 9.60 | – | 5.97 |
| Histidine | His | H | 1.82 | 9.17 | 6.00 | 7.59 |
| Isoleucine | Ile | I | 2.36 | 9.60 | – | 6.02 |
| Leucine | Leu | L | 2.36 | 9.60 | – | 5.98 |
| Lysine | Lys | K | 2.18 | 8.95 | 10.53 | 9.74 |
| Methionine | Met | M | 2.28 | 9.21 | – | 5.74 |
| Phenylalanine | Phe | F | 1.83 | 9.13 | – | 5.48 |
| Proline | Pro | P | 1.99 | 10.60 | – | 6.30 |
| Serine | Ser | S | 2.21 | 9.15 | – | 5.68 |
| Threonine | Thr | T | 2.09 | 9.10 | – | 5.60 |
| Tryptophan | Trp | W | 2.83 | 9.39 | – | 5.89 |
| Tyrosine | Tyr | Y | 2.20 | 9.11 | 10.07 | 5.66 |
| Valine | Val | V | 2.32 | 9.62 | – | 5.96 |
Essential amino acids
Nine amino acids are essential because humans can not synthesize them fast enough to meet their demands.
The essential amino acids are valine, isoleucine, leucine, methionine, phenylalanine, tryptophan, threonine, histidine, and lysine.
The essential amino acids are obtained from foods. Foods from animal sources, e.g., eggs, milk, fish, meat, etc., are complete foods with all the essential amino acids. Foods from plant sources, e.g., wheat, rice, corn, etc., are usually deficient in one or more essential amino acids. So, vegetarians have to eat various vegetarian foods to obtain all the essential amino acids.
- Wheat, rice, and oats are deficient in lysine.
- Corn is deficient in lysine and tryptophan.
- Soy is deficient in methionine.
- Beans are deficient in methionine and tryptophan.
- Peas and peanuts are deficient in methionine
- Almonds and walnuts are deficient in lysine, tryptophan
- Foods from animal sources, e.g., milk, eggs, meat, fish, etc., have all the essential amino acids.


