2.1: Hydrocarbons
- Page ID
- 394104
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Identify hydrocarbons and their subclasses: alkane, alkene, alkyne, and aromatic.
- Write IUPAC name for a skeletal structure and draw the skeletal structure from the IUPAC name for simple hydrocarbons.
- Understand some physical properties like melting points, boiling points, and solubilities of hydrocarbons.
- Predict the reactive sites, i.e., \(\delta\)- (nucleophilic) and \(\delta\)+ (electrophilic) regions in the structures of simple hydrocarbons.
- Identify primary, secondary, tertiary, or quaternary \(\ce{C's}\) and \(\ce{H's}\).
Classification of hydrocarbons
Organic compounds composed of \(\ce{C's}\) and \(\ce{H's}\) only are called hydrocarbons.
The hydrocarbons are divided into two major classes based on the absence or presence of an aromatic ring.
An aromatic ring is a cyclic chain of only sp2-hybridized atoms in planer geometry having \(\pi\)-electrons in the ring equal to 4n+2, where n is a positive integer, i.e., 0, 1, 2, 3, ...,. Benzene is an example of an aromatic ring that is a cycle of six sp2-hybridized \(\ce{C's}\) having six \(\pi\)-electrons, which is a number equal to 4n+2 for n = 1.

Benzene
- Hydrocarbons with at least one aromatic ring in their structure are called aromatic hydrocarbons.
- Hydrocarbons that do not have an aromatic ring in their structure are called aliphatic hydrocarbons. The aliphatic hydrocarbons are subdivided into i) alkanes, ii) alkenes, and iii) alkynes. Figure \(\PageIndex{1}\) illustrates the classification of hydrocarbons.
- Alkanes have all \(\ce{C-C}\) single bonds, i.e., all the \(\ce{C's}\) are sp3-hybridized, e.g., propane ( \(\ce{\small{H}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\)).
- Alkenes have at least one \(\ce{C=C}\) double bond, e.g., propene ( \(\ce{\small{H}-{\underset{\underset{\Large{H}} |}{C}}={\underset{\underset{\Large{H}} |}{C}}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\)).
- Alkynes have at least one \(\ce{C≡C}\) triple bond, e.g., propyne ( \(\ce{\small{H}-{C}≡{C}-\overset{\overset{\Large{H}}|}{\underset{\underset{\Large{H}} |}{C}}\!-H}\)).

Alkanes
Nomenclature of alkanes
Organic compounds have trivial names as well as systematic names. Systematic names are based on the International Union of Pure and Applied Chemistry (IUPAC) recommendations, referred to as IUPAC nomenclature. Table 1 lists stem names that represent the number of \(\ce{C's}\) in a continuous chain of \(\ce{C's}\) (straight chain) or a cyclic chain part of an organic compound.
| # of \(\ce{C’s}\) | Stem name | # of \(\ce{C’s}\) | Stem name | # of \(\ce{C’s}\) | Stem name |
|---|---|---|---|---|---|
| 1 | Meth | 11 | Undec | 30 | Triacont |
| 2 | Eth | 12 | Dodec | 40 | Tetracont |
| 3 | Prop | 13 | Tridec | 50 | Pentacont |
| 4 | But | 14 | Tetradec | 60 | Hexacont |
| 5 | Pent | 15 | Pentadec | 70 | Heptacont |
| 6 | Hex | 16 | Hexadec | 80 | Octacont |
| 7 | Hept | 17 | Heptadec | 90 | Nonacont |
| 8 | Oct | 18 | Octadec | 100 | hect |
| 9 | Non | 19 | Nonadec | ||
| 10 | Dec | 20 | Icos |
Straight chain alkanes
To name a straight chain alkane, the first syllable, i.e., alk- is replaced with the stem name that tells the number of \(\ce{C's}\) in the chain, and the last syllable, i.e., -ane is retained as a suffix in which 'an' tells all bonds are single bonds, and 'e' tells it is a hydrocarbon, i.e., -ane means an alkane.
For example, \(\ce{CH4}\) is called methane, \(\ce{CH3-CH3}\) is called ethane, and \(\ce{CH3-CH2-CH3}\) is called propane, where meth-, eth-, and prop- are the stem names representing one, two, and three \(\ce{C's}\). Table 2 shows the formulas, IUPAC names of some straight-chain alkanes, and some of their physical properties. The physical properties will be discussed in a later section.
| Formula | IUPAC Name | Melting point (oC) | Boiling point (oC) | Density (g/ml) | Physical state at 20 oC |
|---|---|---|---|---|---|
| \(\ce{CH4}\) | Methane | −182 | −162 | 0.000656 | Gas |
| \(\ce{C2H6}\) | Ethane | −183 | −89 | 0.00126 | Gas |
| \(\ce{C3H8}\) | Propane | −188 | −42 | 0.00201 | Gas |
| \(\ce{C4H10}\) | Butane | −138 | 0 | 0.00248 | Gas |
| \(\ce{C5H12}\) | Pentane | −130 | 36 | 0.626 | Liquid |
| \(\ce{C6H14}\) | Hexane | −95 | 69 | 0.659 | Liquid |
| \(\ce{C7H16}\) | Heptane | −91 | 98 | 0.684 | Liquid |
| \(\ce{C8H18}\) | Octane | −57 | 126 | 0.703 | Liquid |
| \(\ce{C9H20}\) | Nonane | −54 | 151 | 0.718 | Liquid |
| \(\ce{C10H22}\) | Decane | −30 | 174 | 0.730 | Liquid |
| \(\ce{C11H24}\) | Undecane | 196 | 0.740 | Liquid | |
| \(\ce{C12H26}\) | Dodecane | −10 | 216 | 0.749 | Liquid |
| \(\ce{C13H28}\) | Tridecane | −5.4 | 235 | 0.756 | Liquid |
| \(\ce{C14H30}\) | Tetradecane | 5.9 | 253 | 0.763 | Liquid |
| \(\ce{C15H32}\) | Pentadecane | 10 | 270 | 0.769 | Liquid |
| \(\ce{C16H34}\) | Hexadecane | 18 | 287 | 0.773 | Liquid |
| \(\ce{C17H36}\) | Heptadecane | 22 | 303 | 0.777 | Liquid |
| \(\ce{C18H38}\) | Octadecane | 28 | 317 | 0.781 | Solid |
| \(\ce{C19H40}\) | Nonadecane | 32 | 330 | 0.785 | Solid |
| \(\ce{C20H42}\) | Icosane | 37 | 343 | 0.789 | Solid |
A regular zigzag line, also called a straight chain, usually represents the hydrocarbons, but it may be a wiggle waggle because of rotation allowed around each \(\ce{C-C}\) single bond. Figure \(\PageIndex{2}\) shows the semi-condensed formulas of decane presented in five different configurations and the corresponding skeletal formulas. The terminal \(\ce{C}\) in a chain is the carbon at the ends connected with only one other \(\ce{C}\). The internal carbon in a chain is the \(\ce{C}\) connected with at least two other \(\ce{C's}\).
- A continuous \(\ce{C}\) chain starts from a terminal carbon and reaches the other terminal \(\ce{C}\) through the connected continuous \(\ce{C-C}\) single bonds.
- A straight-chain hydrocarbon has all the internal \(\ce{C's}\) connected with only two other \(\ce{C's}\), no matter how much wiggle waggle the chain may be.








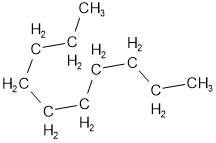

Branched alkanes
If at least one \(\ce{C}\) in an alkane is connected with three or four other \(\ce{C's}\), it is a branched alkane. The parent chain is the longest continues \(\ce{C}\) chain. The smaller chains branching from the parent chain are called branches, substituents, or alkyl groups. R- represents a general alkyl group. Rules for naming the branched alkanes are the following.
- Find the most extended continuous \(\ce{C}\) chain, called the parent chain. Name it by using the stem name that tells the number of \(\ce{C's}\) in it with the suffix -ane that means it is an alkane.
 Not
Not 
- Name the branch chain by using the stem name that tells the number of \(\ce{C's}\) in the continues \(\ce{C}\) chain of the branch with the suffix -yl that tells it is a branch. Use the name of the branch as a prefix to the name of the parent chain.

- If there are two or more longest chains of equal length, choose the parent chain with the highest number of branches on it.
 not
not 
- If there is more than one branch, arrange their names alphabetically as prefixes to the name of the parent chain.
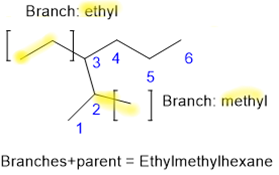
- If there is more than one branch with the same name, list it once with the prefix di-, tri-, tetra-, etc., added to the branch name to represent two, three, four, etc. of the same branches. The alphabetization of the branch names is based on the branch name without the prefixes di-, tri-, tetra-, etc. For example, dimethyl, trimethyl, and tetramethyl are all alphabetized based on the 'm' of methyl, not based on the 'd' or 't' first alphabets of the prefixes.
 not
not 
- Number the \(\ce{C's}\) of the parent chain starting from the end that gives the lowest number to whichever branch appears first on the parent chain.
 not
not
- If numbering from left or right has the same number for the first branch, start the numbering from the side that gives a lower number to the branch that comes alphabetically first.
 not
not 
- List the number to which the branch is attached to the parent chain before the branch name, separated by a hyphen. This is the IUPAC name of the alkane.
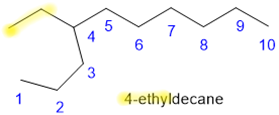 ,
, , and
, and 
- If two or more branches have the same name, list their numbers in increasing order, separated by commas, and the final number separated by a hyphen from the branch name.
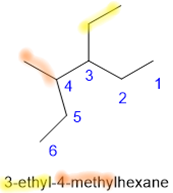
- If two branches are on the same \(\ce{C}\), use the same number twice, i.e., once for each branch.

- If two or more branches have the same name, list their numbers in increasing order, separated by commas, and the final number separated by a hyphen from the branch name.
- If a branch is further branched, name it as if it is a parent chain following the above rules, except that i) the suffix of the brach is -yl instead of -ane, ii) insert a number from which the beach is attached to the parent chain before -yl, iii) Enclose the branch name in small brackets and place it as a prefix to the name of the parent chain, iv) insert a number before the bracket to which the beach is attached on the parent change, as shown in the following example.

- The terminal \(\ce{C's}\), i.e., the \(\ce{C's}\) with only one single bond with another \(\ce{C}\) are primary carbon.
- The \(\ce{H's}\) bonded to primary \(\ce{C's}\) are primary hydrogen.
- The \(\ce{C's}\) with two single bonds with other \(\ce{C's}\) are secondary carbon.
- The \(\ce{H's}\) bonded to secondary \(\ce{C's}\) are secondary hydrogen.
- The \(\ce{C's}\) with three single bonds with other \(\ce{C's}\) are tertiary carbon.
- The \(\ce{H's}\) bonded to tertiary \(\ce{C's}\) are tertiary hydrogen.
- The \(\ce{C's}\) with four single bonds with other \(\ce{C's}\) are quaternary carbon.
The quaternary \(\ce{C's}\) do not have any \(\ce{H}\) bonded to them. The following figure labels the primary, secondary, and tertiary carbons and hydrogen with different colors.

Cycloalkanes
An alkane with one ring of \(\ce{C's}\) in its structure is called cycloalkane. IUPAC rules for naming cycloalkanes are the following.
- If there is one ring of \(\ce{C's}\) without any substituent on it, it is named by using the stem name that tells the number of \(\ce{C's}\) in the ring with prefix cyclo- and suffix -ane, as shown in the following examples.

- If there is only one branch or a substituent on the ring of \(\ce{C's}\), then the ring supplies the parent name, and the branch name is added as a prefix, as for alkanes but without any number preceding the branch name. Some examples of IUPAC names of branched cycloalkanes are shown below.
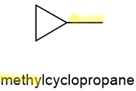 ,
, 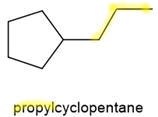 , and
, and 
- If there are two substitutes, they are listed alphabetically, preceded by location number, as in the case of alkanes. The numbering of \(\ce{C's}\) in the chain starts from the point of attachment of the substituent that comes alphabetically first. The numbering goes clockwise or counterclockwise, whichever gives the second substitute the lowest number, as shown in the following examples.
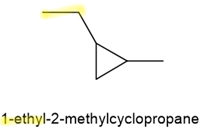 ,
,  , and
, and 
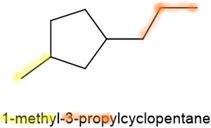
- If there are more than two substituents, they are listed as prefixes in alphabetic order as for alkanes. The ring \(\ce{C's}\) attached to one of the substituents is assigned number 1, and the numbering is continued clockwise or counterclockwise so that the addition of numbers assigned to the substituents is the lowest possible. Some examples are shown below.
 ,
,  , and
, and 
Sometimes the ring name is treated as a branch with the last syllable changed from -ane to -yl, e.g., cyclopropane becomes cyclopropyl as a branch. This change is applied particularly when the chain attached to the ring has more number of \(\ce{C's}\) than the number of \(\ce{C's}\) in the ring.
Cycloalkanes with two alkyl groups on different carbons have two configurations: i) both alkyl groups pointing in the same direction are called 'cis' orientation, and ii) the two bulky groups pointing in the opposite direction are called 'trans' orientation, as shown below for the case of 1,2-dimethyl cyclopropane and 1-ethyl-3-methylcyclohexane examples.
 cis-1,2-dimethylcyclopropane
cis-1,2-dimethylcyclopropane trans-1,2-dimethylcyclopropane
trans-1,2-dimethylcyclopropane cis-1-ethyl-3-methylcyclohexane
cis-1-ethyl-3-methylcyclohexane trans-1-ethyl-3-methylcyclohexane
trans-1-ethyl-3-methylcyclohexaneThe cis- and trans- configuration can not inter-convert by rotation around \(\ce{C-C}\) bond as the rotation is restricted by the carbon bridge in the case of cycloalkanes. Therefore, the cis- and trans-configuration of cycloalkane are isomers of each other, i.e., the same formula but different compounds. For example, cis-1,2-dimethylcyclopropane has a boiling point of 37 C, and its isomer, i.e., trans-1,2-dimethylcylopropane has a boiling point of 28 oC. This phenomenon, i.e., cis-, trans- isomerism, is common in all cyclic compounds.
Bonding and physical properties of alkanes
Bonding
The general formula of straight chain and branched alkanes is \(C_{n}H_{(2n+2)}\), and that of cycloalkanes having one ring is \(C_{n}H_{(2n)}\) where n is a positive integer. All \(\ce{C's}\) in alkanes are sp3 hybridized, i.e., tetrahedral geometry with all bond angles about 109.5o, as illustrated in Figure \(\PageIndex{3}\) for the case of ethane molecule. The bond energy for \(\ce{C-C}\) bond is ~3.8 eV and for \(\ce{C-H}\) bond is ~4.4 eV.

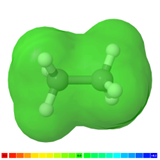
Polarity
The electronegative difference for the \(\ce{C-H}\) bond is 0.4, which falls in the nonpolar bond category. The electrostatic potential map of a molecule shows \(\delta\)+ region in blue, \(\delta\)- region in red, and neutral or nonpolar region in green color. Alkanes are nonpolar, as shown in green in the electrostatic map of ethane in Figure \(\PageIndex{3}\). The strong \(\ce{C-C}\) and \(\ce{C-H}\) bonds make the alkanes stable and less reactive. The nonpolar nature of alkanes makes them more inert chemically.
Intermolecular forces and boiling points
Alkanes are nonpolar molecules where the only intermolecular interaction is London dispersion forces. Straight-chain alkanes are a homologous series.
As the molar mass, and, consequently, the surface area and the number of electrons in the electron cloud, gradually increase, the London dispersion forces increase, resulting in a gradual increase in melting and boiling points, as shown in Table 2.
The first four members for the alkane series, i.e., \(\ce{CH4}\) to \(\ce{C4H10}\) are gases, the next from \(\ce{C5H12}\) to \(\ce{C17H36}\) are liquid and the larger alkanes are wax or soft solids.
Natural gas is the primary source of methane and ethane. Petroleum, which in the crude oil form is a viscous liquid composed of thousands of organic compounds, primarily hydrocarbons, is the primary source of alkanes and also the main source of organic raw materials. The differences in the boiling points allow the segregation of crude oil into different fractions, as illustrated in Figure \(\PageIndex{4}\).

Solubility
Alkanes are not soluble in water because alkanes are nonpolar, and water is a polar solvent. Alkanes are soluble in each other or other nonpolar solvents. Alkanes float on water because they are less dense than water. The density of liquid alkanes varies in the range of 0.6 g/ml to 0.8 g/ml, which is less than the density of water, which is ~1 g/ml at room temperature. Petroleum and crude oil are mainly composed of alkanes, which is why oil spills make a layer of oil float on the water's surface.
Changes in physical properties with branching
The physical properties of branched alkanes are almost the same as that of straight chain alkanes, except that:
- the polling point increases as the molar mass increases, and
- for the same molar mass, the boiling point decreases as the branching increases, as shown in Table 1.
| Number of \(\ce{C's}\) | Name | Condensed formula | Boiling point |
|---|---|---|---|
| 5 | Pentane | \(\ce{CH3CH2CH2CH2CH3}\) | 36oC |
| 6 | Hexane | \(\ce{CH3CH2CH2CH2CH2CH3}\) | 69oC |
| 6 | 2-Methylpentane | \(\ce{(CH3)2CHCH2CH2CH3}\) | 60oC |
| 6 | 2,2-Dimethylbutane | \(\ce{(CH3)3CCH2CH3}\) | 50oC |
| 7 | Heptane | \(\ce{CH3CH2CH2CH2CH2CH2CH3}\) | 98oC |
Straight-chain alkanes are long and cylindrical, as illustrated in Figure \(\PageIndex{5}\). Branching makes them spherical, resulting in less contact area between molecules, fewer London dispersion forces, and lower boiling points. Note that the effect of molar mass is dominant over that of branching.
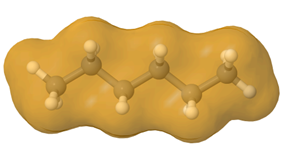
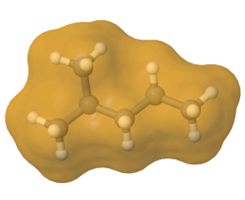
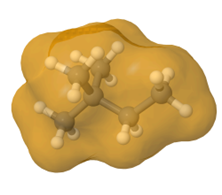
Properties of cycloalkanes
The physical properties of cycloalkanes are almost similar to alkanes, except that bonds have to bend to acquire ring-shape that causes angle strain. Further, the molecule can not acquire the most stable configuration due to no or limited rotation around \(\ce{C-C}\) single bond. Therefore, the cycloalkanes, mainly the smaller three or four-member rings, are relatively unstable. Except for cyclopropane, there is a limited rotation possible around \(\ce{C-C}\) bond that allows the molecule to acquire bent-shape, rather than a planer shape that releases some of the strains, as shown by models in Figure \(\PageIndex{6}\). The strain decreases, and the stability increases in this order: cyclopropane > cyclobutane > cyclopentane > cyclohexane < cycloheptane > cyclooctane. Notably, the five and six-membered rings, e.g., cyclopentane and cyclohexane, have little or no strain, so they are the most common cyclic compounds found in nature.





Some uses of alkanes
The first four members, i.e., methane, ethane, propane, and butane, are gases used as fuels. The next four are components of gasoline, and higher than that are components of kerosene and diesel, used as fuels.
Alkanes and other hydrocarbons are part of almost all organic compounds but are not so common in pure form in living things. Some examples of uses in biological systems are the following. Long-alkanes having eighteen or more \(\ce{C,s}\) are semisolids found in waxes as in vaseline used in ointments and cosmetics. The waxes are found on surfaces of leaves and fruits to protect against water loss, prevent the leaching of essential minerals by rain, and protect against bacteria, fungi, and harmful insects. Alkanes are also found in some pheromones, e.g., sand bees use tricosane (\(\ce{C23H48}\)), pentacosane (\(\ce{C25H53}\)), and heptacosane (\(\ce{C27H56}\)) to identify a mate.
Alkenes
Nomenclature of alkenes
The procedure for IUPAC naming alkenes is the same as alkanes, except for the following changes.
- The suffix to the stem name of the parent chain is -ene, where 'en' tells its an alkene, and 'e' means it is a hydrocarbon, e.g., \(\ce{CH2=CH2}\) is ethene.
- A location number of the first \(\ce{C}\) of \(\ce{C=C}\) bond is inserted between the stem- name and -ene suffix, separated by hyphens, as in the following example, except for propene that does not need to show the location number.

3-ethylpent-2-ene
- The parent chain numbering starts from the end that gives the lowest number to the first \(\ce{C}\) of \(\ce{C=C}\) bond.
- If the first \(\ce{C}\) of \(\ce{C=C}\) bond receives the same number from either side, then the rule for alkane applies, i.e., start numbering from the side that gives the lower number to the branch that appears first, as shown in the following examples.

3-ethyl-6-methylhept-3-ene, \(\xcancel{\text{5-ethyl-2-methylhept-4-ene}}\)

4-ethyl-2-methylhex-3-ene, \(\xcancel{\text{3-ethyl-5-methylhept-3-ene}}\)
- If two or more double bonds exist, the suffix -ene is changed to -diene, triene-, etc. The rest of the nomenclature remains the same.

buta-1,3-diene

2-methylbuta-1,3-diene
- In cycloalkenes, the \(\ce{C's}\) of \(\ce{C=C}\) are #1 and #2 and do not need to show the location number.
- If there is a branch on cycloalkene, the \(\ce{C'}\) of \(\ce{C=C}\) bond are #1 and #2, and the numbering is continued clockwise or counterclockwise to give the lowest number to the branch, as shown in the following examples.
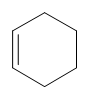
cyclohexene
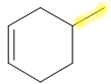
4-methylcyclohex-1-ene
- there are two alkyl groups, one on each \(\ce{C}\) of \(\ce{C=C}\) bond (or third or fourth group different from the other group on the same \(\ce{C}\)) then a descriptor E indicating cis, or Z indicating the trans orientation of the bulkier groups, preceded by the location number of the first \(\ce{C}\) of the \(\ce{C=C}\) bond, is added as a prefix enclosed within brackets.

(2E)-pent-2-ene
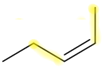
(2Z)-pent-2-ene
- if the double bond is in a branch, the -ene suffix is replaced with -enyl, where 'en' tells it is an alkene and 'yl' means it is a branch.
In the older system:
- The parent chain may not be the longest, but the parent chain is the longest chain counting the double bond, and
- The location number of the double bond is inserted before the stem name, e.g., but-2-ene (\(\ce{CH3CH=CHCH3}\)) is written as 2-butene in the old IUPAC nomenclature.
Some common names are still being used in the chemical literature and industries. For example, ethylene, propylene, isobutylene, and isoprene are often used in place of IUPAC names ethene, propene, 2-methylpropene, and 2-methylbuta-1,3-diene.
| Structure and IUPAC name |
ethene |
prop-1-ene |
2-methylprop-1-ene |
2-methylbuta-1,3-diene |
| Common name | ethylene | propylene | isobutylene | isoprene |
Bonding and physical properties of alkenes
Alkenes are nonpolar compounds with only London dispersion forces as intermolecular forces, like alkanes. Therefore, the physical properties of alkenes are similar to alkanes with the same \(\ce{C}\) skeleton, i.e., they are insoluble in water, soluble in nonpolar solvents, and have densities lower than that of water. The significant difference between alkanes and alkenes is the \(\ce{C=C}\) bond in alkenes described below.
bonding
The general formula of alkenes with one double bond is \(C_{n}H_{(2n)}\), where n is a positive integer. The \(\ce{C's}\) in \(\ce{C=C}\) bond are sp2 hybridized with trigonal planer geometry around each of the sp2 \(\ce{C's}\) with bond angles around 120o, as illustrated in Figure \(\PageIndex{7}\). Except for \(\ce{C=C}\), all other \(\ce{C's}\) in alkenes are sp3 hybridized, i.e., tetrahedral geometry with all bond angles about 109.5o. Although alkenes are nonpolar overall, the Electrostatic potential map in Figure \(\PageIndex{7}\) shows a red region where \(\pi\)-electrons are located above and below the \(\sigma\) bond and slightly blush region where \(\ce{H's}\) of sp2 \(\ce{C's}\) are located. These \(\delta {-}\) and \(\delta {+}\) regions impart reactivity to alkenes that will be described in later sections.
The general formula of an alkane is \(C_{n}H_{(2n+2)}\), where n is a positive integer. One degree of unsaturation is two less \(\ce{H's}\) than in an alkane of the same carbon structure. For example, one ring in cycloalkane or one double bond in alkenes reduces two \(\ce{H's}\), one unsaturation degree. The degree of unsaturation is also known as the index of hydrogen deficiency (IHD). Remember, if a halogen with one bond is added to an alkane, it replaces one \(\ce{H}\), an \(\ce{O}\) with two bonds does not change the number of \(\ce{H's}\), and a \(\ce{N}\) with three bonds add one \(\ce{H}\). Based on this information, the degree of unsaturation, i.e., number of double bonds + rings, can be calculated by the following formula.
\[IHD = (C's + 1) - {\frac{H's-N's+X's}{2}}\nonumber \]
, where \(\ce{IHD}\) is an index of hydrogen deficiency, which is equivalent to the number of \(\pi\) bonds + rings, \(\ce{C's}\) is the number of \(\ce{C}\) atoms, \(\ce{H's}\) is the number of \(\ce{H}\) atoms, \(\ce{N's}\) is the number of \(\ce{N}\) atoms, and \(\ce{X's}\) is number of halogen atoms in the molecular formula.
If oxygen is in the molecule, it is not included in the IHD calculation.
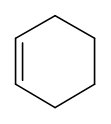
What is the degree of saturation in cyclohexane (\(\ce{C6H10}\)?
Solution
\(\ce{C's}\) = 6, \(\ce{H's}\) = 10, \(\ce{N's}\) = 0, \(\ce{X's}\) = 0.
\(IHD = (C's + 1) - {\frac{H's-N's+X's}{2}}\) = \((6 + 1) - {\frac{10-0+0}{2}}\) = 2, which is one \(\pi\)-bond and one ring as shown below.
Substituents attached to sp2 hybridized \(\ce{C's}\) of alkenes are also called vinylic. The bond energy for \(\ce{C=C}\) bond is ~7.4 eV which is less than two times of ~3.8 eV of \(\ce{C-C}\) bond, and the vinylic \(\ce{=C-H}\) bond is ~4.8 eV, which is stronger than alkane \(\ce{-C-H}\)~4.4 eV. Comparison ~7.4 eV for \(\ce{C=C}\) bond vs 3.8 eV for \(\ce{C-C}\) bond shows that, although a double bond ((\(\sigma\) + \(\pi\)) is stronger than a single bond, a \(\pi\) bond is weaker than a \(\sigma\) bond.


If there are two alkyl groups, one on each \(\ce{C}\) of a double bond, they can be pointing in the same direction, called cis orientation, or in the opposite direction, called trans orientation, as shown in the Figure below for the case of but-2-ne. The cis and trans alkenes of the same molecular formula are different compounds related as isomers of each other. This is because three is no rotation around \(\ce{C=C}\) bond without breaking and re-making the \(\pi\) bond.
 cis-but-2-ene, IUPAC name: (2Z)-but-2-3n3
cis-but-2-ene, IUPAC name: (2Z)-but-2-3n3
Cis trans isomerism plays a vital role in biological systems. For example, fatty acids are significant components of fats and vegetable oils. Fatty acid contains a long alkyl chain attached to a carboxylic acid group. The alkyl chain is usually alkane in the cases of animal fats or includes one or more double bonds, almost always in the cis configuration in the cases of vegetable oils and fish oil. For example, models of steric acid with no double bonds, oleic acid with one cis double bond, and linoleic acid with two cis double bonds are shown below.
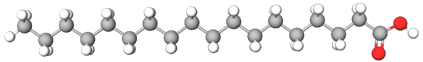


The cis-double bonds change the long cylindrical shape of fatty acids to a bent or more spherical shape with less contact area with the neighboring molecules. It results in lower intermolecular forces and melting points than the same chain with a trans or no double bond. That is why vegetable oils with more cis-double bonds are liquids, while animal fats with little to no double bonds are solids at room temperature. Another example is vitamin A having five trans double bonds, as shown in the model drawing below. Vitamin A leads to retinol synthesis, which plays a crucial role in our vision based on switching one of its double bonds between cis and trans configurations.

Model of vitamin A molecule with five trans double bonds.
Polarity and chemical reactivity
Although \(\ce{C=C}\) bond is nonpolar, the electrostatic potential map of ethene in Figure \(\PageIndex{7}\) shows there is an electron-rich region (red color in the map) above and below the axis joining the nuclei. This is because the \(\sigma\) bond is along the axis of nuclei, and the \(\pi\) bond places electrons above and below the \(\sigma\) bond. Molecules with negative charge or electron-rich (\(\delta\)-) regions, as in the case of \(\ce{C=C}\) bond in alkene, are nucleophiles. Molecules with positive charge or electron-deficient (\(\delta\)+) region are electrophiles. Chemical reactions between nucleophiles and electrophiles are facilitated by the attraction between the opposite charges that allow them to come closer to each other for bond-making and bond-breaking to happen.
The term acid is used for electrophile when \(\delta\)+ part is a proton, and the base is used in the place of nucleophile when \(\delta\)- region is a lone pair of electrons interacting with a proton.
Alkenes uses and importance in biological systems
Alkenes are among the raw materials for the synthesis of several organic chemicals. Mainly, ethene and propene are the raw material for synthesizing commercially important polymers: polyethylene and polypropylene.
Ethene is a ripening agent for fruits. It allows fruit growers to pick fruits while they are green and less susceptible to bruising and then treat them with ethene gas for ripening when ready for sale. Lycopene, -a red pigment in tomatoes, and carotene, -a yellow pigment in carrots, are polyenes, shown below.
Terpenes are a class of natural products having a general formula \(\ce{(C_{5}H_{8})n}\), where n is a positive integer. Isoprene (\(\ce{(C_{5}H_{8})}\), i.e., 2-methylbuta-1,3-diene is the building block of terpene.

isoprene, IUPAC name: 2-methylbuta-1,3-diene
Terpenoids are derivatives of terpenes in which heteroatoms/functional group is added. Natural rubber, essential oils, and steroids are examples of terpenes or terpenoids. They play important roles in living things, e.g., in defense against diseases. Some examples of terpenes and terpenoids are shown below, with the isoprene skeleton highlighted.



Alkynes
Nomenclature
The IUPAC nomenclature of alkynes followes the same rule as alkenes, except for the following changes.
- The suffix to the stem name is -yne where 'yn' tells it is an alkyne, and 'e' means it is a hydrocarbon, e.g., \(\ce{CH≡CH}\) is ethyne.
- If a double and a triple bond are in the same molecule, they are treated equally. One exception is when the double receives the same number as the triple bond counted from the other end of the parent chain; preference is given to the double bond, as shown in the following examples.


Note: in the second example, the double bond received a lower number than the triple bond. The common name of ethyne is acetylene, which is also accepted as its IUPAC name.
Bonding and physical properties of alkynes
Alkynes are nonpolar compounds with only London dispersion forces as intermolecular forces, like alkenes and alkanes. Therefore, the physical properties of alkynes are similar to alkanes with the same \(\ce{C}\) skeleton. They are insoluble in water, soluble in nonpolar solvents, and have lower densities than water's. The significant difference is the presence of a triple bond in alkynes described below.
Bonding
The general formula of alkyne with one triple bond is \(C_{n}H_{(2n-2)}\), where n is a positive integer. The \(\ce{C's}\) in \(\ce{C≡C}\) bond are sp hybridized with a linear geometry around each of the sp \(\ce{C's}\) with bond angles 180o, as illustrated in Figure \(\PageIndex{8}\). Substituents attached to sp hybridized \(\ce{C's}\) of alkynes are also called acetylenic. The bond energy for \(\ce{C≡C}\) bond is ~10 eV which is about 2.5 times stronger than that of \(\ce{C-C}\) bond. Due to the cylindrical shape of two \(\pi\)-bond clouds, there is free rotation possible around a \(\ce{C≡C}\) bond, but due to the linear geometry, it means less.


polarity and chemical reactivity
Although \(\ce{C≡C}\) bond is nonpolar, the electrostatic potential map of ethyne in Figure \(\PageIndex{8}\) shows there is an electron-rich region (red color in the map) around the axis joining the nuclei. This is due to \(\pi\)-bonding electrons around the \(\sigma\) bond. The \(\pi\) bonds provide \(\delta\)- region in alkynes, making them nucleophiles, like alkenes. The \(\ce{H's}\) are also blue in the electrostatic potential map of ethyne, showing that the \(\ce{C-H}\) bond is polar with \(\delta {+}\) charge on \(\ce{H}\).
The \(\ce{C-H}\) bond is nonpolar with an electronegativity difference of 0.4 between \(\ce{C}\) and \(\ce{H}\). The green color in the electrostatic potential map of ethane reflects it. However, the \(\ce{C-H}\) bond becomes shorter, stronger, and polar with a change in the hybridization of the \(\ce{C}\) from sp3 to sp2 and sp. It means the electronegativity of \(\ce{C}\) increases with a change in the hybridization from sp3 to sp2 and sp. The reason is that the sp orbital is wider and shorter due to more s-orbital character (50% s-character) than sp2 orbital (33% s-character), which, in turn is wider and shorter the sp3 orbital (25% s-character), as illustrated in Fig. 7. The wide and shorter orbitals place the electron closer to the \(\ce{C}\) nucleus for a stronger attraction to the nucleus, which means higher electronegativity.



Uses of alkynes
 Alkynes are less common in biological systems or the chemical industry than alkenes and alkanes. The first member, i.e., acetylene, is used in oxyacetylene torches for cutting and welding metals, as illustrated in the figure on the right. Acetylene is also a starting material for several chemicals used in the chemical industry.
Alkynes are less common in biological systems or the chemical industry than alkenes and alkanes. The first member, i.e., acetylene, is used in oxyacetylene torches for cutting and welding metals, as illustrated in the figure on the right. Acetylene is also a starting material for several chemicals used in the chemical industry.
Aromatic hydrocarbons
Aromatic hydrocarbons contain a planer cycle or ring (not bent or puckered) in their structure with all atoms sp2-hybridized and \(\pi\text{-electrons in the ring} = 4n +2\), where n is a positive integer. Benzene is an example of an aromatic ring that will be described here. Although aromatic hydrocarbons have systematic names like other hydrocarbons, some alternate and familiar names are so widely used that they are accepted as equivalent to IUPAC names.
Nomenclature of benzene derivatives
- The name of the six sp2 hybridized \(\ce{C}\)-chain is benzene. It is used as the parent name for the substituted benzene rings.
- If there is only one substituent, i.e., mono-substituted benzene, use the branch name as a prefix to the parent name 'benzene' without numbering.
- If the substituent is methyl (\(\ce{-CH3}\)), the parent name 'toluene' is often used for it, and if the substituent is ethenyl (\(\ce{-CH=CH2}\)), it is also called styrene. A few examples are given below.
 benzene
benzene
 toluene or methylbenzene
toluene or methylbenzene
 ethylbenzene
ethylbenzene
 propylbenzene
propylbenzene
 ethenylbenzne or styrene
ethenylbenzne or styrene
- If there are two substituents (di-substituted benzene), start numbering the chain from the first substituent and go clockwise or counterclockwise so that the second substituent receives the lower number. List the substitutes alphabetically, preceded by their number, as prefixes to the parent name 'benzene.'
- If one of the two substitutes is methyl (\(\ce{-CH3}\)), start numbering the chain from the point of attachment of methyl and go clockwise or counterclockwise so that the second substitute receives the lower number. List the second substituent preceded by its number as a prefix to the parent name 'toluene.'
- If there are two methyls (\(\ce{-CH3}\)) substituents, the parent name 'xylene' is often used. Numbering starts from one of the methyls and goes clockwise or counterclockwise, so the second methyl receives the lower number. List the numbers as prefixes to the parent name 'xylene,' numbers separated by commas from each other and by a hyphen from the parent name, as usual.
- Alternate to the numbers, the word 'ortho' or 'o' for a 1,2 relationship, 'meta' or 'm' for a 1,3 relationship, and 'para' or 'p' for a 1,3 relationship of two substituents on a benzene ring are also accepted. A few examples are the following.
 1,4-diethylbenzene or p-diethylbenzene
1,4-diethylbenzene or p-diethylbenzene
 1-ethyl-3-methylbenzne or 3-ethyltoluene
1-ethyl-3-methylbenzne or 3-ethyltoluene
 1,2-xylene or o-xylene
1,2-xylene or o-xylene
 1,3-xylene or m-xylene
1,3-xylene or m-xylene
 1,4-xylene or p-xylene
1,4-xylene or p-xylene
- If there are more than two substituents (poly-substituted benzene), start numbering the chain from the first substituent and go clockwise or counterclockwise so that the substituents receive an overall lower number. List the substitutes alphabetically, preceded by their number, as prefixes to the parent name 'benzene.'
- If one substituent is methyl (\(\ce{-CH3}\)), start numbering from the point of attachment of methyl (\(\ce{-CH3}\)) and go as usual to assign the lower overall number to the rest of the substituents, and use the parent name 'toluene.'
 2,4-diethyl-1-methylbenzne or 2,4-diethyltoluene
2,4-diethyl-1-methylbenzne or 2,4-diethyltoluene
Bonding and physical properties of aromatic hydrocarbons
Aromatic hydrocarbons are nonpolar compounds with only London dispersion forces as intermolecular forces, like other hydrocarbons. Benzene has low solubility in water (~1.8 g/L at room temperature), melts at 5.53 oC, and boils at 80.1 oC, which are comparable to that of cyclohexane (immiscible in water, 6.5 oC, and boiling point is 80.7 oC). The primary difference is resonance resulting in significant stabilization of benzene compared to any other hydrocarbon, as described below.
Bonding
All \(\ce{C's}\) in the benzene ring are sp2 hybridized, i.e., trigonal planer geometry with all bond angles about 120o, as illustrated in Figure \(\PageIndex{10}\). All \(\ce{C's}\) in benzene ring and \(\ce{H's}\) or other substituents attached to them are in one plane. Substituents attached to the \(\ce{C's}\) of the benzene ring are also called phenylic. The bond energy for \(\ce{C\overset\cdots{-}C}\) bond in benzene ring is ~5.4 eV which is between a \(\ce{C-C}\) and a \(\ce{C=C}\) bond. the phenylic \(\ce{C-H}\) bond is ~4.9 eV, almost the same as in alkenes. The \(\ce{C\overset\cdots{-}C}\) bond in the benzene ring is (140 pm), i.e., about in the middle of a single and a double bond. This is explained by benzene having two equal resonance contributors in which a single and double bond alternates and the resonance hybrid has a 50% \(\pi\)-bond.


polarity and chemical reactivity
Although benzene is nonpolar, the electrostatic potential map of benzene in Figure \(\PageIndex{10}\) shows there is a circle of an electron-rich region (red color in the map) above and below the ring. This is due to the delocalized \(\pi\)-bond extending over all \(\ce{C's}\) of the benzene ring. The resonance in a cycle of aromatic rings imparts significantly more resonance stabilization, called aromatic stabilization, than in any other hydrocarbon.
Although the \(\delta^-\) region of delocalized elections makes benzene a nucleophile, its nucleophilic character is weak because donating these electrons causes a loss of aromatic stabilization. Therefore benzene needs stronger electrophiles to react with than an alkene and follows a different reaction route than an alkene does.
Benzene increases the risk of leukemia and other blood disorders. Polycyclic aromatic hydrocarbons (PAHs) contain two or more benzene rings fused edge to edge. Naphthalene has two benzene rings fused. Naphthalene is used in mothballs. Anthracene has there benzene rings linked together. Anthracene is used in the manufacture of dyes. Phenanthrene is also three benzene rings fused. Phenanthrene is known to cause cancer, i.e., it is a carcinogen. Benzo[a]pyrene has five benzene rings connected. Benzo[a]pyrene is a potent carcinogen found in tobacco smoke, barbecued meat, and automobile exhaust.
 naphthalene
naphthalene anthracene
anthracene phenanthrene
phenanthrene benzo[a]pyrene
benzo[a]pyreneUses of aromatic hydrocarbons
Benzene is used as a raw material for synthesizing several chemicals. Some familiar aromatic ring-containing compounds include explosives, e.g., trinitrotoluene (TNT), common medicines, e.g., aspirin, acetaminophen, ibuprofen, sulfanilamide, and flavoring agents, e.g., vanillin, shown below.
 2,4,6-trinitrotoluene, TNT
2,4,6-trinitrotoluene, TNT 2-acetyloxybenzoic acid
2-acetyloxybenzoic acid
(Aspirin)
 N-(4-hydroxyphenyl)acetamide
N-(4-hydroxyphenyl)acetamide
(Acetaminophene)
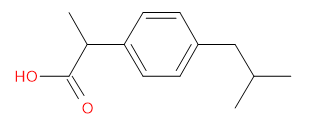 2-[4-(2-methylpropyl)phenyl]propanoic acid
2-[4-(2-methylpropyl)phenyl]propanoic acid
(Ibuprofen)
 4-aminobenzenesulfonamide
4-aminobenzenesulfonamide
(Sulfanilamide)
 4-hydroxy-3-methoxybenzaldehyde
4-hydroxy-3-methoxybenzaldehyde
(Vanilin)





