8.3: Half-life of radioisotopes
- Page ID
- 372950
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The half-life (t1/2) of a radioisotope is the time it takes for half of the sample to decay.
It tells the rate of decay of the radioisotope – the faster the rate of decay, the shorter the half-life.
General features of the half-life
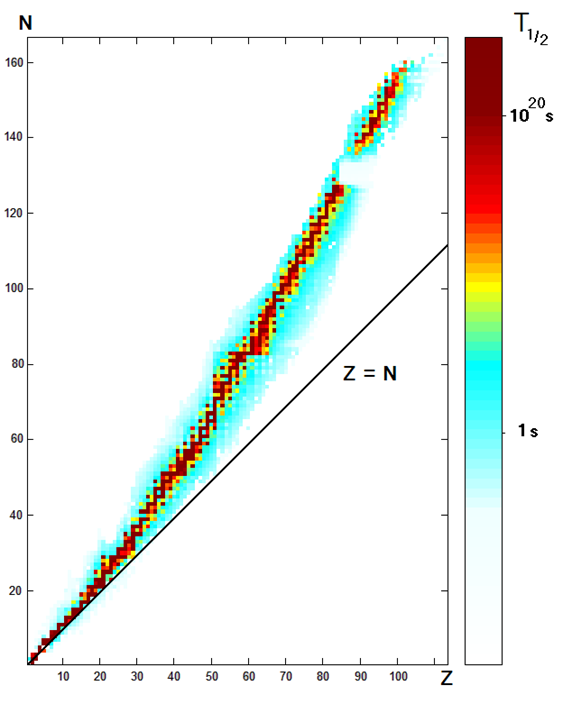
- The half-life is different for different nucleoids, as shown in Fig. 8.3.1, and Table 1. It varies from a fraction of a second to more than 1020 s, i.e., more than 3 trillion years.
- The farther a nucleoid is away from the stable nucleoid (shown by black dots in Fig. 8.3.1), the less stable it is, and the faster it decays.
- The half-life is independent of concentration, temperature, and pressure, i.e., the t1/2 is a characteristic constant of a radioisotope.
- Natural radioactive isotopes usually have a longer half-life, e.g., t1/2 carbon-14 is 5730 years and uranium-235 is 7.0 x 108 years.
- The radioisotopes used for imaging and treatment in medical sciences are usually synthesized and have a short half-life so that they may not persist in the body for an unnecessarily long time. For example, phosphorous-32, iodine-131, and technetium-99m have half-lives of 14.3 days, 8.1 days, and 6.0 hours, respectively.
| Radioisotope | Symbol | Half-life | Use |
|---|---|---|---|
| Carbon-14 | \(\ce{_6^14C}\) | 5730 years | Radioisotope dating |
| Hydrogen-3 | \(\ce{_1^3H}\) | 12.3 years | Radioisotope dating |
| Potassium-40 | \(\ce{_19^40K}\) | 1.3 x 109 years | Radioisotope dating |
| Rhenium-187 | \(\ce{_75^187Re}\) | 4.3 x 1010 years | Radioisotope dating |
| Uranium-238 | \(\ce{_92^238U}\) | 4.5 x 109 years | Radioisotope dating |
| Uranium-235 | \(\ce{_92^235U}\) | 7.0 x 108 years | Nuclear reactor fuel |
| Cobalt-60 | \(\ce{_27^60Co}\) | 5.3 years | Medical (external radiation source) |
| Iodine-131 | \(\ce{_53^131I}\) | 8.1 days | Medical |
| Iron-59 | \(\ce{_26^59Fe}\) | 45 days | Medical |
| Molybdenum-99 | \(\ce{_42^99Mo}\) | 67 hours | Medical |
| Sodium-24 | \(\ce{_11^24Na}\) | 15 hours | Medical |
| Technetium-99m | \(\ce{_43^{99m}Te}\) | 6 hours | Medical |
| Phosphoros-32 | \(\ce{_15^32P}\) | 14.3 dsys | Medical |
Decay curve of radioisotopes
During each successive half-life, half of the initial amount will radioactively decay, as illustrated in Fig. 8.3.2. for the case of phosphorous-32 that decays with a half-life of 14.3 days by the following nuclear reaction
\[\ce{_15^32P -> _16^32S + _{-1}^{0}{e}}\nonumber\]
Suppose there is 100 mg of the phosphorous-32 in the beginning; 50 mg will be left behind after 14.3 days, i.e., after 1 half-life; and 25 mg will be left after 28.6 days, i.e., after 2 half-lives. A negligible amount of the parent isotope phosphorous-32 is left after 9 half-lives.
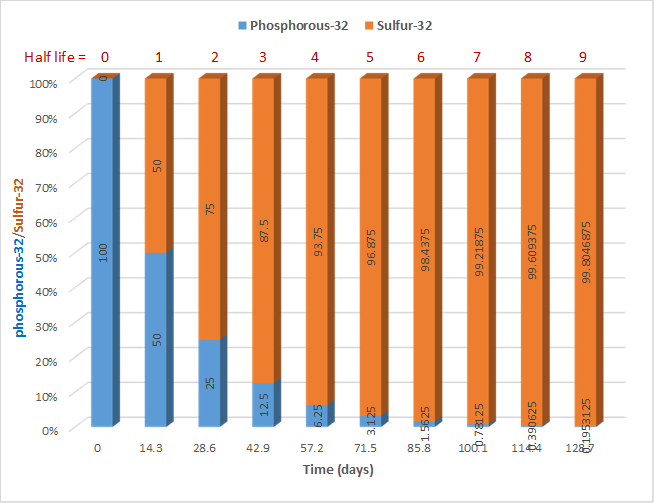
The amount of a radioisotope remaining after the given time can be calculated from the known initial amount and time spent, by the following formula:
\[m_{f}=m_{i}(0.5)^{n}\nonumber\]
where mi is the initial amount, mf is the final amount, and n is the number of half-lives passed. The formula works even if the number of half-lives is not a whole number.
If 50.0 mg of iodine-131 was injected for medical treatment, how many milligrams will be left after 40.5 days? (Half-life of iodine-131 is 8.1 days)
Solution
Given: mi = 50.0 mg, Time = 40.5 days, Desired ? mf
The equality: 1 half-live = 8.1 days, gives the following conversion factors. \[\frac{1 \text { half-life }}{8.1 \text { days }} \quad\text{and}\quad \frac{8.1 \text { days }}{1 \text { half-life }}\nonumber\]
For calculating the half-lives, multiple the given time with the conversion factor that cancels the time:
\[n=40.5 \cancel{\text { days }} \times \frac{1 \text { half-life }}{8.1 \cancel{\text { days }}}=5 \text { half-lives }\nonumber\]
For calculating the amount left, plug in the values in the formula:
\[m_{f}=m_{i}(0.5)^{n}=50.0 \mathrm{mg}(0.5)^{5}=1.56 \mathrm{~mg}\nonumber\]
Radioisotope dating
Natural radioactivity is used to establish the age of objects of archeological, anthropological, or historic interest. All living objects have carbon in their composition. Carbon-14 is a radioactive isotope of carbon with a half-life of 5730 years. Carbon-14 is produced by the transmutation of nitrogen-14 upon neutron bombardment by cosmic rays, as illustrated in Fig. 8.3.3. Its concentration in a carbon source for the living organism remains almost constant because its decay counterbalances its production by cosmic rays. Living organisms continuously replenish carbon, so the carbon-14 concentration remains almost constant as long as the object is alive. After the object dies, the carbon-14 decreases with time, reducing to half after one half-life. The carbon-12 isotope is not radioactive, so its concentration remains constant. Measurement of the carbon-14/carbon-12 ratio allows calculating the age of the object after its death. The age of early civilizations, like the Indus valley civilization examples shown in Fig. 8.3.4 were determined by the carbon-14 dating method.

Because the carbon-14 decreases with time, the object older than 20,000 years of age does not have sufficient carbon-14 left to determine their age accurately. Other radioisotopes with a longer half-life, e.g., uranium-238 with a half-life of 4.5 x 109 years, are used to determine the age of ancient objects. For example, the age of rock samples from the moon, as shown in Fig. 8.3.5 was determined by uranium-238 radioisotope dating.



