7.2: The pressure-volume relationship
- Page ID
- 371600
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Pressure-volume relationship
Consider a gas in a cylinder with a piston pushing it down due to massive objects placed on it and due to outside air pressure, as illustrated in Fig. 7.2.1, The gas molecules strike the piston surface, applying pressure upward equal to the downward pressure applied by the piston that keeps the piston stationary. Increasing the pressure on the piston, e.g., by adding more weight to it, causes the piston to move down, reducing the gas volume. The gas molecules have less distance to travel before striking the piston surface which increases the collision frequency and causes the gas pressure to increase until becomes equal to the outside pressure.
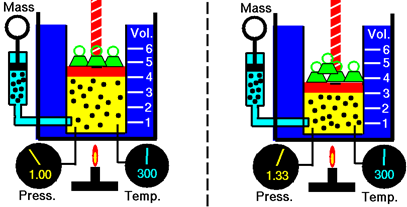
When a change in one parameter causes a change in another, the parameters are related. When an increase in one parameter causes a decrease in another, the two are inversely proportional to each other. Robert Boyle studied the quantitative relationship between the volume and pressure of the gas, keeping the quantity of gas and the temperature constant. The research concluded in a law called Boyle’s law, which states that:
The volume of a gas is inversely proportional to the pressure of the gas provided the temperature and the amount of the gas remain constant.
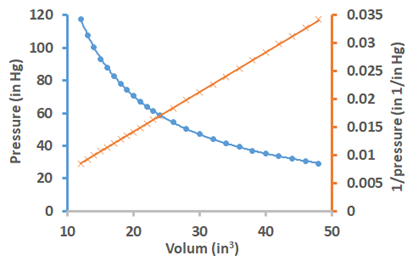
Fig. 7.2.2 illustrates the results of Boyle’s experiment. The mathematical form of Boyle’s law is: \[V \propto \frac{1}{P}\nonumber\], or \[V=\frac{\mathrm{k}}{P}\nonumber\], or \[PV=\mathrm{k}\nonumber\], where k is a consant. The graph of volume versus pressure is curvilinear, but the graph of volume versus the reciprocal of pressure is a linear graph showing the inverse proportionality between the volume and the pressure. Since the product PV is a constant, it implies that: \[P_{1} V_{1}=P_{2} V_{2}={k}\nonumber\], i.e., a product of initial pressure (P1) and initial volume (V1) is equal to the product of final pressure (P2) and final volume (V2) of gas provided the quantity of the gas and temperature does not change.
The pressure of a 1.32 L sample of SO2 gas at 0.532 atm is increased to 1.231 atm. Calculate the new volume of the gas if the temperature and the quantity of the gas remain the same?
Solution
Given: P1 = 0.532 atm, P2 = 1.231 atm, V1 = 1.32 L V2 = ?
Formula: \(P_{1} V_{1}=P_{2} V_{2}={k}\), rearrange to isolate the desired variable: \(V_{2}=\frac{P_{1} V_{1}}{P_{2}}\).
Plug in the values in the rearranged formula and calculate: \(V_{2}=\frac{0.532 \mathrm{~atm} \times 1.32 \mathrm{~L}}{1.231 \mathrm{~atm}}=0.570 \mathrm{~L}\)
An oxygen tank holds 20.0 L of oxygen at a pressure of 10.0 atm. What is the final pressure when the gas is released and occupies a volume of 200 L?
Solution
Given: V1 = 20.0 L, V2 = 200 L, P1 = 10.0 atm P2 = ?
Formula: \(P_{1} V_{1}=P_{2} V_{2}={k}\), rearrange to isolate the desired variable: \(P_{2}=\frac{P_{1} V_{1}}{V_{2}}\).
Plug in the values in the rearranged formula and calculate: \(P_{2}=\frac{10.0 \mathrm{~atm} \times 20.0 \mathrm{~L}}{200 \mathrm{~L}}=1.00 \mathrm{~atm}\).
Breathing process
Boyle’s law explains the mechanism of the breathing process. Lungs are elastic structures like balloons placed in the thoracic cavity, as illustrated in Fig. 7.2.3. The diaphragm muscle makes a flexible floor and ribs surround the cavity.
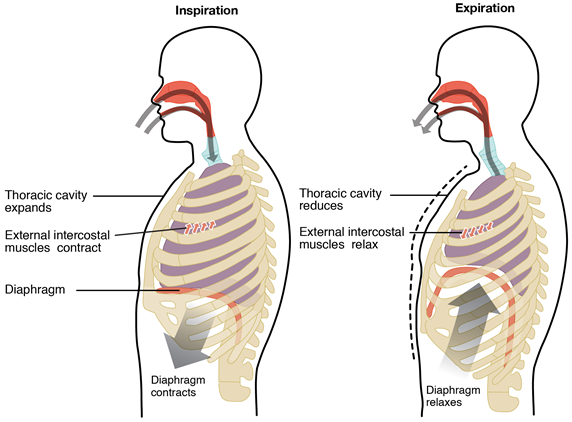
Inhalation
The inhalation or the inspiration process starts when the diaphragm contract and move down and the rib muscles contract, expanding the thoracic cavity. Volume increases, the air pressure decreases inside the inside thoracic cavity and the atmospheric air flows into the lungs until the pressure in the lungs is equal to the outside pressure.
Exhalation
The exhalation or the expiration process starts when the diaphragm expands and moves upwards, and the rib muscles relax, contracting the thoracic cavity. Volume decreases and the air pressure increases inside the thoracic cavity that pumps the air out of the lungs into the atmosphere.


