5.1: Introduction to solution
- Page ID
- 372207
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
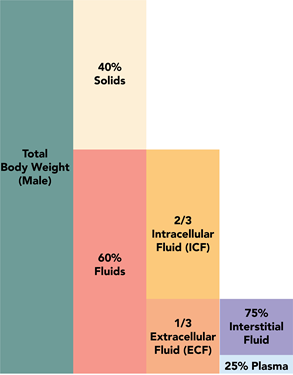
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Solutions are all around us, e.g., air, seawater, body fluids, metal alloys are solutions. Fig. 5.1.1 illustrates that air is a mixture of nitrogen, oxygen, carbon dioxide, and some other gases; Fig. 5.1.2 illustrates that seawater is a mixture of water, chloride, sodium, sulfate, magnesium, and some other ions, and Fig. 5.1.3. illustrates that about 60% of the human body is composed of solutions called body fluids.


What is a solution?
- A solution is a homogeneous mixture of two or more pure substances.
- The substance that is in a large amount in the solution is called the solvent.
- The substance that is in smaller amounts in a solution is called the solute.
For example, the air is a solution in which nitrogen is the solvent, and water is the solvent in seawater and body fluids. Oxygen, carbon dioxide, and water vapors are solutes in the air; and sodium, chloride, sulfate, magnesium, and some other ions are solutes in seawater.
Types of solution
The solutions are generally classified in two ways: i) based on the physical state of the solution and the solute, and ii) based on the particle size of the solute.
Types of solution based on the physical state of the solution and the solute
The solutions can be classified based on the physical state of the solution, solvent, and solute. For example, the air is gas in a gas solution; carbonated water is a gas in a liquid solution; vinegar is a liquid in a liquid solution; metal alloys are solid in solid solutions. Table 5.1.1 lists the major types of solutions, solvents, and primary solutes in them.
| Type | Example | solvent | Primary solute |
|---|---|---|---|
| Gas in gas | Air | Nitrogen | Oxygen |
| Gas in liquid | Carbonated water | Water | Carbon dioxide |
| Liquid in liquid | Vinegar | Water | Acetic acid |
| Solid in liquid | Seawater | Water | Sodium chloride |
| Solid in solid | Brass | Copper | Zinc |
Types of solutions based on the particle size of the solute
A solution is a homogeneous mixture comprising smaller component/s called solute/s of small molecules or ions comparable in size to the molecules of a larger component called the solvent.
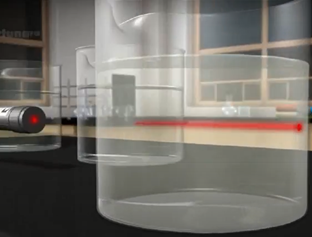
For example, NaCl dissolved in water is a solution. The solute is almost uniformly distributed in the solvent, making a homogeneous mixture. The solute does not separate by filtration or by a semipermeable membrane but can be separated by some other physical process. For example, the distillation process separates a solid in a liquid or a liquid into a liquid solution. The solution is transparent, though it may be colored. A light passing through a solution is not visible, as shown in Fig. 5.1.4.

A suspension is a heterogeneous mixture of solvent and solute particles of larger than 10,000 Å.
For example, muddy water is a suspension. If the suspension is allowed to stand, the suspended particles settle down and separate. The suspended particles can be filtered out. Some medicines, e.g., milk of magnesia, are suspensions. It is instructed to shake just before administering medicine to re-suspend the settled suspension.
A colloid falls between a solution and a suspension. The colloidal particles are larger molecules like proteins or groups of molecules or ions.
Unlike a suspension, the colloids usually do not settle if allowed to stand. The colloidal particles can not be filtered but can be separated by a semipermeable membrane. When a light beam passes through a colloid, it scatters by the colloid particles, called the Tyndall effect, and becomes visible, as shown in Fig. 5.1.4.
Examples of colloids include:
- fog and clouds that are liquid water droplets dispersed in air;
- smoke that is solid carbon particles dispersed in air;
- whipped cream that is air dispersed in a liquid;
- styrofoam is a gas dispersed in a solid; and
- ager medium that is liquid dispersed in a solid medium.
Water –a universal solvent
Water (H2O) is an essential substance for life. It covers more than 70% of the earth’s surface (Fig. 5.1.5), and it comprises more than 60% of the human body (5.1.3). In addition to being the most abundant solvent, water is a universal solvent because it is a polar molecule with a partial negative charge on oxygen and a partial positive charge on hydrogen atoms as shown in Fig. 5.1.6. The polarity of water molecules allows them to interact with other water molecules as well as with other polar compounds through dipole-dipole interactions and with other ions through ion-dipole interactions. These interactions help to dissolve a lot of polar and ionic compounds that are in and around us.


How does water dissolves polar and ionic compounds?
Water molecules establish electrostatic interaction, called hydrogen bonding, through the partial +ve end of one molecule with a partial –ve end of a neighboring molecule. These interactions impart unique properties to water, like its relatively higher boiling point and melting point compared to other substances of similar molecular weight. Other polar substances have similar interactions, e.g., ethanol has hydrogen bonding similar to water as illustrated in Fig. 5.1.7.
When water mixes with other polar substances, like ethanol, some of the hydrogen bonding between water molecules replace with similar hydrogen bonding with ethanol molecules. Since the electrostatic potential energy is similar, the natural tendency to go towards more dispersion drives the dispersion of ethanol molecules uniformly in water resulting in the solution.



Ionic compounds are held together by electrostatic forces between opposite ions, i.e., ionic bonds. When an ionic compound is added to water, water molecules surround the cation and establish ion-dipole interaction by orienting their partial -ve end to the cation. Similarly, water molecules establish ion-dipole interaction with anions by orienting their partial +ev end towards the anion, as illustrated in Fig. 5.1.8
The ion-dipole interactions, along with nature’s tendency to disperse the particles, are usually strong enough to overcome the ionic bonds, dissociate the compounds into ions, and disperse them almost uniformly in the water.
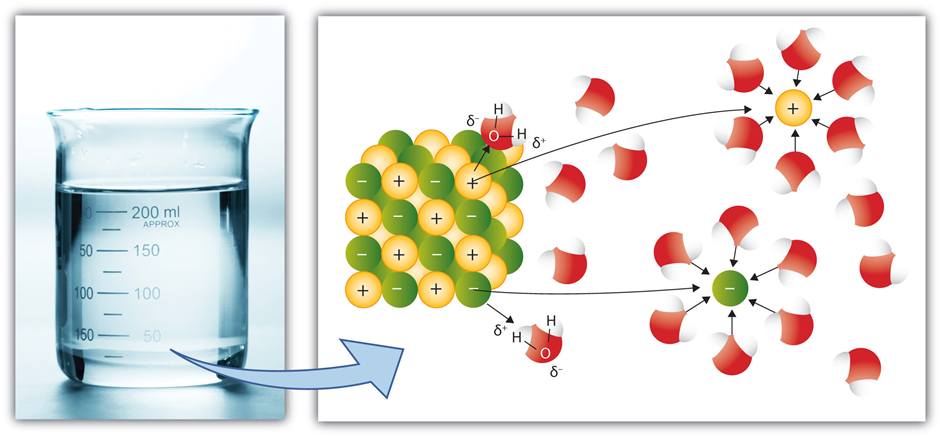
The separation of the cations from the anions of the ionic compound is called dissociation.
The formation of a layer of water molecules around ions, driven by ion-dipole interactions, is called hydration.
Non-polar substances, like vegetable oil or gasoline, do not dissolve in water. The molecules in non-polar substances have only London dispersion forces. They easily dissolve in non-polar solvents like hexane or carbon tetrachloride that have similar London dispersion forces among their molecules.
The fact that ionic and polar substances dissolve in polar solvents and non-polar substances dissolve in non-polar solvents of similar intermolecular interactions is called “like dissolves like.”


