7.3: Exothermic and Endothermic Reactions
- Page ID
- 86225
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Use bond dissociation energies to calculate enthalpy change or heat of reaction.
- Determine if a chemical process is exothermic or endothermic.
Endothermic and Exothermic Reactions
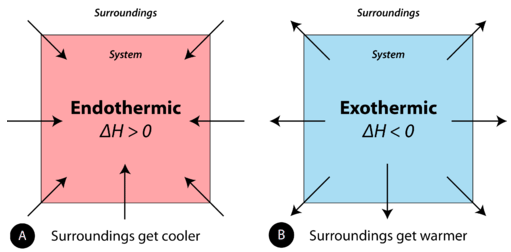
Endothermic and exothermic reactions can be thought of as having energy as either a reactant of the reaction or a product. Endothermic reactions require energy, so energy is a reactant. Heat flows from the surroundings to the system (reaction mixture) and the enthalpy of the system increases (\(\Delta H\) is positive). As discussed in the previous section, heat is released (considered a product) in an exothermic reaction, and the enthalpy of the system decreases (\(\Delta H\) is negative).
In the course of an endothermic process, the system gains heat from the surroundings and so the temperature of the surroundings decreases (gets cold). A chemical reaction is exothermic if heat is released by the system into the surroundings. Because the surroundings is gaining heat from the system, the temperature of the surroundings increases. See Figure \(\PageIndex{1}\).
Endothermic Reaction: When \(1 \: \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \: \text{mol}\) of carbon dioxide, \(177.8 \: \text{kJ}\) of heat is absorbed. Because the heat is absorbed by the system, the \(177.8 \: \text{kJ}\) is written as a reactant. The \(\Delta H\) is positive for an endothermic reaction.

\[\ce{CaCO_3} \left( s \right) \rightarrow \ce{CaO} \left( s \right) + \ce{CO_2} \left( g \right) \: \: \: \: \: \Delta H = +177.8 \: \text{kJ}\]
Exothermic Reaction: When methane gas is combusted, heat is released, making the reaction exothermic. Specifically, the combustion of \(1 \: \text{mol}\) of methane releases 890.4 kilojoules of heat energy. This information can be shown as part of the balanced equation in two ways. First, the amount of heat released can be written in the product side of the reaction. Another way is to write the \(\Delta H\) information with a negative sign, \(-890.4 \: \text{kJ}\).
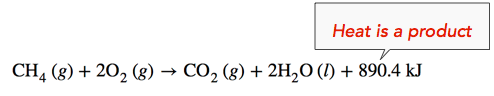
\[\ce{CH_4} \left( g \right) + 2 \ce{O_2} \left( g \right) \rightarrow \ce{CO_2} \left( g \right) + 2 \ce{H_2O} \left( l \right) \: \: \: \: \: \Delta H = -890.4 \: \text{kJ}\]
Is each chemical reaction exothermic or endothermic?
- CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ) + 213 kcal
- N2(g) + O2(g) + 45 kcal → 2NO(g)
Solution
- Because energy (213 kcal) is a product, energy is given off by the reaction. Therefore, this reaction is exothermic.
- Because energy (45 kcal) is a reactant, energy is absorbed by the reaction. Therefore, this reaction is endothermic.
Is each chemical reaction exothermic or endothermic?
- H2(g) + F2(g) → 2HF (g) + 130 kcal
- 2C(s) + H2(g) + 5.3 kcal → C2H2(g)
- Answer
-
a. The energy (130 kcal) is produced, hence the reaction is exothermic
b. The energy (5.3 kcal) is supplied or absorbed to react, hence, the reaction is endothermic
Energy Diagrams
Endothermic and exothermic reactions can be visually represented by energy-level diagrams like the ones in Figure \(\PageIndex{2}\). In endothermic reactions, the reactants have higher bond energy (stronger bonds) than the products. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products. This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\). For an endothermic chemical reaction to proceed, the reactants must absorb energy from their environment to be converted to products.
In an exothermic reaction, the bonds in the product have higher bond energy (stronger bonds) than the reactants. In other words, the energy of the products is lower than the energy of the reactants, hence is energetically downhill, shown in Figure \(\PageIndex{2B}\). Energy is given off as reactants are converted to products. The energy given off is usually in the form of heat (although a few reactions give off energy as light). In the course of an exothermic reaction, heat flows from the system to its surroundings, and thus, gets warm.
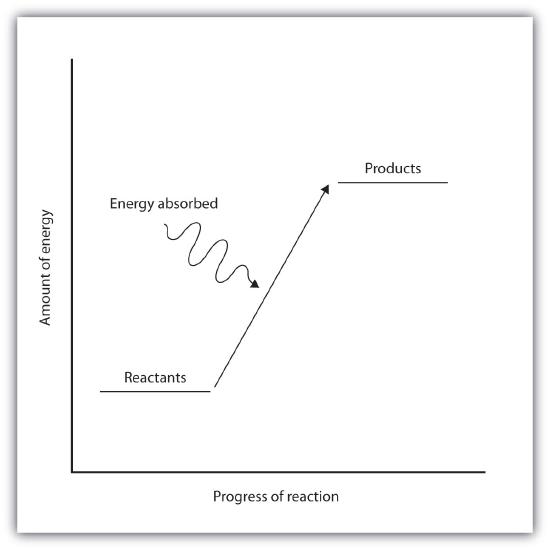
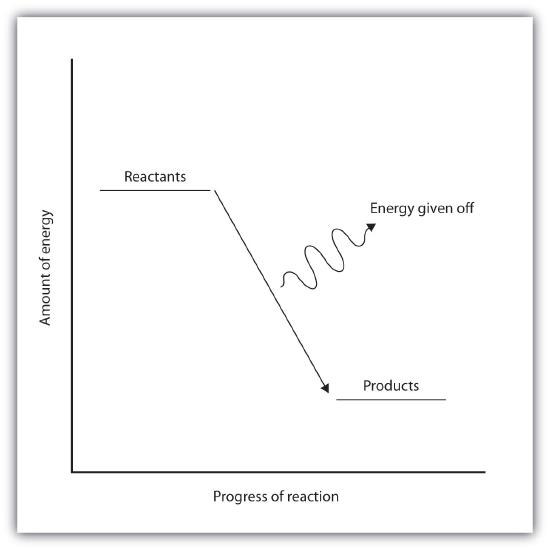
| Endothermic Reactions | Exothermic Reactions |
|---|---|
| Heat is absorbed by reactants to form products. | Heat is released. |
| Heat is absorbed from the surroundings; as a result, the surroundings get cold. | Heat is released by the reaction to surroundings; surroundings feel hot. |
| \(\Delta H_{rxn}\) is positive | \(\Delta H_{rxn}\) is negative |
| The bonds broken in the reactants are stronger than the bonds formed in the products. | The bonds formed in the products are stronger than the bonds broken in the reactants. |
| The reactants are lower in energy than the products. | The products are lower in energy than the reactants. |
| Represented by an "uphill" energy diagram. | Represented by an "downhill" energy diagram |
Concept Review Exercises
- What is the connection between energy and chemical bonds?
- Why does energy change during the course of a chemical reaction?
- Two different reactions are performed in two identical test tubes. In reaction A, the test tube becomes very warm as the reaction occurs. In reaction B, the test tube becomes cold. Which reaction is endothermic and which is exothermic? Explain.
- Classify "burning paper" as endothermic or exothermic processes.
Answers
- Chemical bonds have a certain energy that is dependent on the elements in the bond and the number of bonds between the atoms.
- Energy changes because bonds rearrange to make new bonds with different energies.
- Reaction A is exothermic because heat is leaving the system making the test tube feel hot. Reaction B is endothermic because heat is being absorbed by the system making the test tube feel cold.
- "Burning paper" is exothermic because burning (also known as combustion) releases heat
Key Takeaways
- Atoms are held together by a certain amount of energy called bond energy.
- Energy is required to break bonds. Energy is released when chemical bonds are formed because atoms become more stable.
- Chemical processes are labeled as exothermic or endothermic based on whether they give off or absorb energy, respectively.

