17E: Lipids
- Page ID
- 384617
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Fatty Acids
Concept Review Exercises
-
Give an example of each compound.
f- saturated fatty acid
- polyunsaturated fatty acid
- monounsaturated fatty acid
-
Why do unsaturated fatty acids have lower melting points than saturated fatty acids?
Answers
-
- stearic acid (answers will vary)
- linoleic acid (answers will vary)
- palmitoleic acid (answers will vary)
-
Unsaturated fatty acids cannot pack as tightly together as saturated fatty acids due to the presence of the cis double bond that puts a “kink” or bend in the hydrocarbon chain.
Exercises
Classify each fatty acid as saturated or unsaturated and indicate the number of carbon atoms in each molecule.
- palmitoleic acid
- myristic acid
- linoleic acid
Classify each fatty acid as saturated or unsaturated and indicate the number of carbon atoms in each molecule.
- stearic acid
- oleic acid
- palmitic acid
-
Write the condensed structural formula for each fatty acid.
- lauric acid
- palmitoleic acid
- linoleic acid
-
Write the condensed structural formulas for each fatty acid.
- oleic acid
- α-linolenic acid
- palmitic acid
-
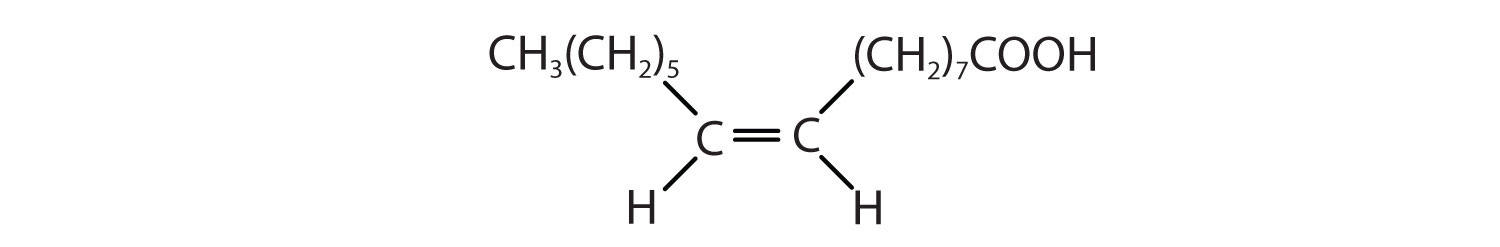
Arrange these fatty acids (all contain 18 carbon atoms) in order of increasing melting point. Justify your arrangement.
-
-
Arrange these fatty acids (all contain 16 carbon atoms) in order of increasing melting point. Justify your arrangement.
- CH3(CH2)14COOH
-
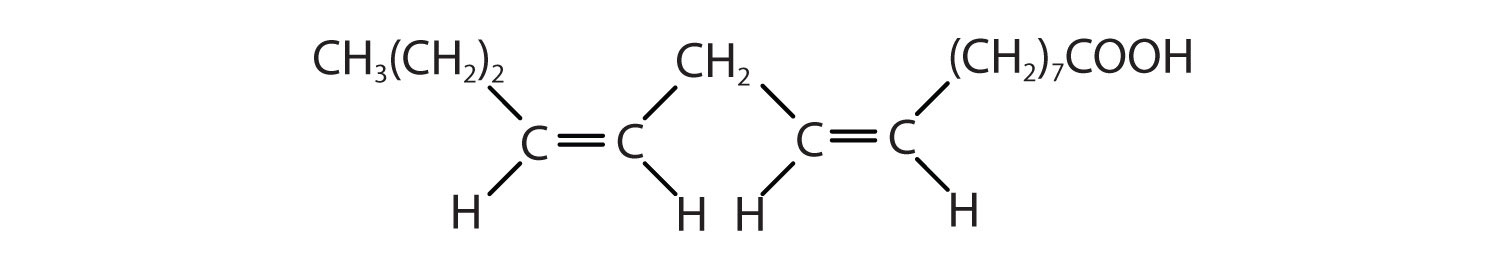
-
Answers
-
- unsaturated; 16 carbon atoms
- saturated; 14 carbon atoms
- unsaturated; 18 carbon atoms
-
- CH3(CH2)10COOH
- CH3(CH2)5CH=CH(CH2)7COOH
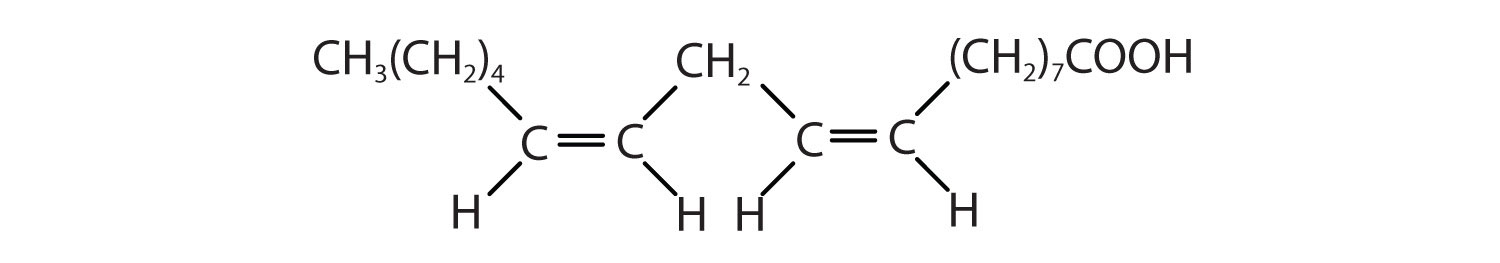
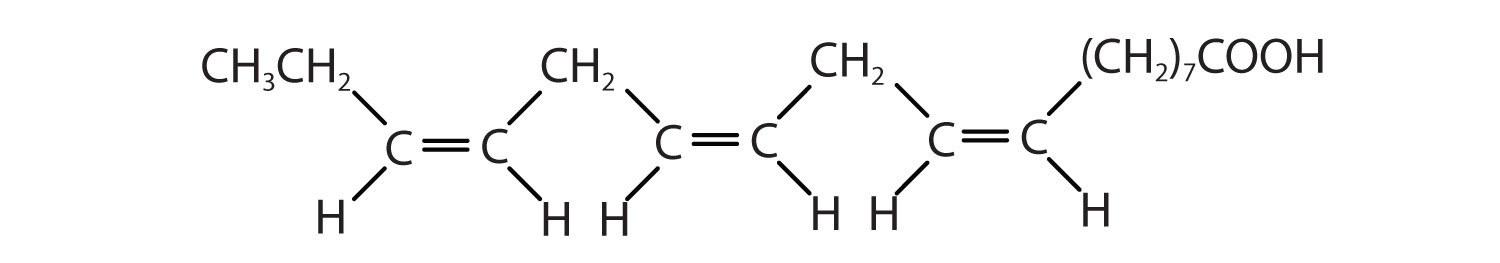
- CH3(CH2)3(CH2CH=CH)2(CH2)7COOH
-
c < a < b; an increase in the number of double bonds will lower the melting point because it is more difficult to closely pack the fatty acids together.
17.2 Fats and Oils
Concept Review Exercises
-
What functions does fat serve in the body?
-
Which of these triglycerides would you expect to find in higher amounts in oils? In fats? Justify your choice.
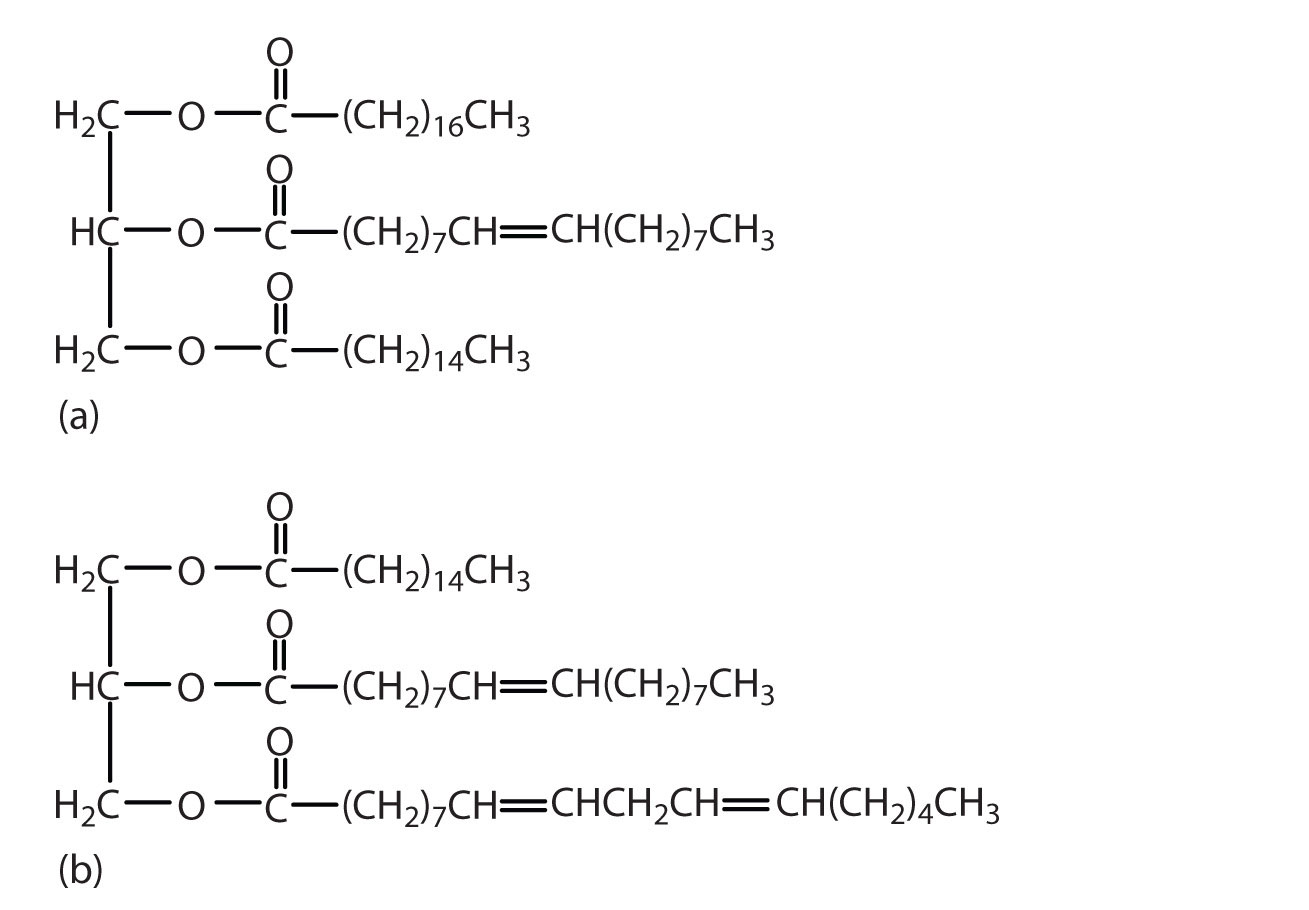
Answers
-
Fats provide energy for living organisms. They also provide insulation for body organs and transport fat-soluble vitamins.
-
The triglyceride on the left is expected to be present in higher amounts in fats because it is composed of a greater number of saturated fatty acids. The triglyceride on the right is expected to be present in higher amounts in oils because it is composed of a greater number of unsaturated fatty acids.
Exercises
-
Draw the structure for each compound.
- trimyristin
- a triglyceride likely to be found in peanut oil
-
Draw the structure for each compound.
- tripalmitin
- a triglyceride likely to be found in butter
-
Draw structures to write the reaction for the complete hydrogenation of tripalmitolein (Table \(\PageIndex{1}\) for the condensed structure of palmitoleic acid). Name the product formed.
-
Draw structures to write the reaction for the complete hydrogenation of trilinolein (Table \(\PageIndex{1}\) for the condensed structure of linoleic acid). Name the product formed.
-
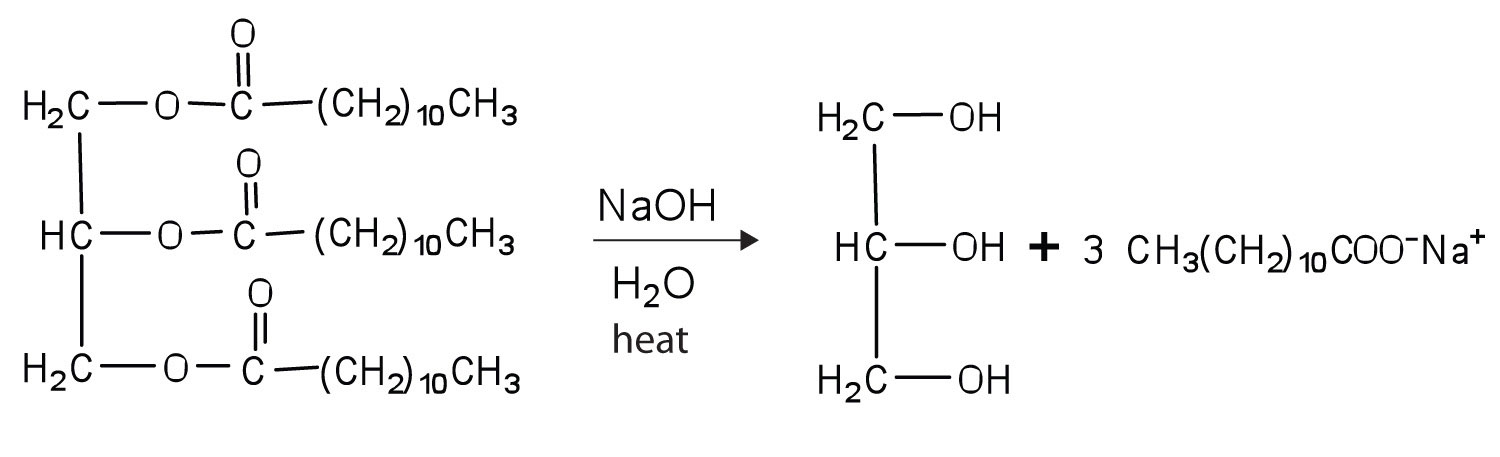
Draw structures to write the reaction for the hydrolysis of trilaurin in a basic solution (Table \(\PageIndex{1}\) for the condensed structure of lauric acid).
-
Draw structures to write the reaction for the hydrolysis of tristearin in a basic solution (Table \(\PageIndex{1}\) for the condensed structure of stearic acid).
-
- What compounds with a disagreeable odor are formed when butter becomes rancid?
- How are these compounds formed?
- How can rancidity be prevented?
-
- What compound with a disagreeable odor is formed when unsaturated fatty acids react with oxygen in the atmosphere?
- How can this process be prevented?
Answers
1 
-
- smaller carboxylic acids, such as butyric, caprylic, and capric acids
- These compounds are formed by the hydrolysis of the triglycerides found in butter.
- Rancidity can be prevented by covering the butter (to keep out moisture) and storing it in a refrigerator. (Cold temperatures slow down hydrolysis reactions.)
17.3 Membranes and Membrane Lipids
Concept Review Exercises
-
Name the structural unit that must be present for a molecule to be classified as a
- phospholipid.
- glycolipid.
- sphingolipid.
-
Why is it important that membrane lipids have dual character—part of the molecule is hydrophilic and part of the molecule is hydrophobic?
-
Why do you suppose lecithins (phosphatidylcholines) are often added to processed foods such as hot cocoa mix?
Answers
-
- a phosphate group
- a saccharide unit (monosaccharide or more complex)
- sphingosine
-
The dual character is critical for the formation of the lipid bilayer. The hydrophilic portions of the molecule are in contact with the aqueous environment of the cell, while the hydrophobic portion of the lipids is in the interior of the bilayer and provides a barrier to the passive diffusion of most molecules.
-
Lecithin acts as an emulsifying agent that aids in the mixing of the hot cocoa mix with water and keeps the cocoa mix evenly distributed after stirring.
Exercises
-
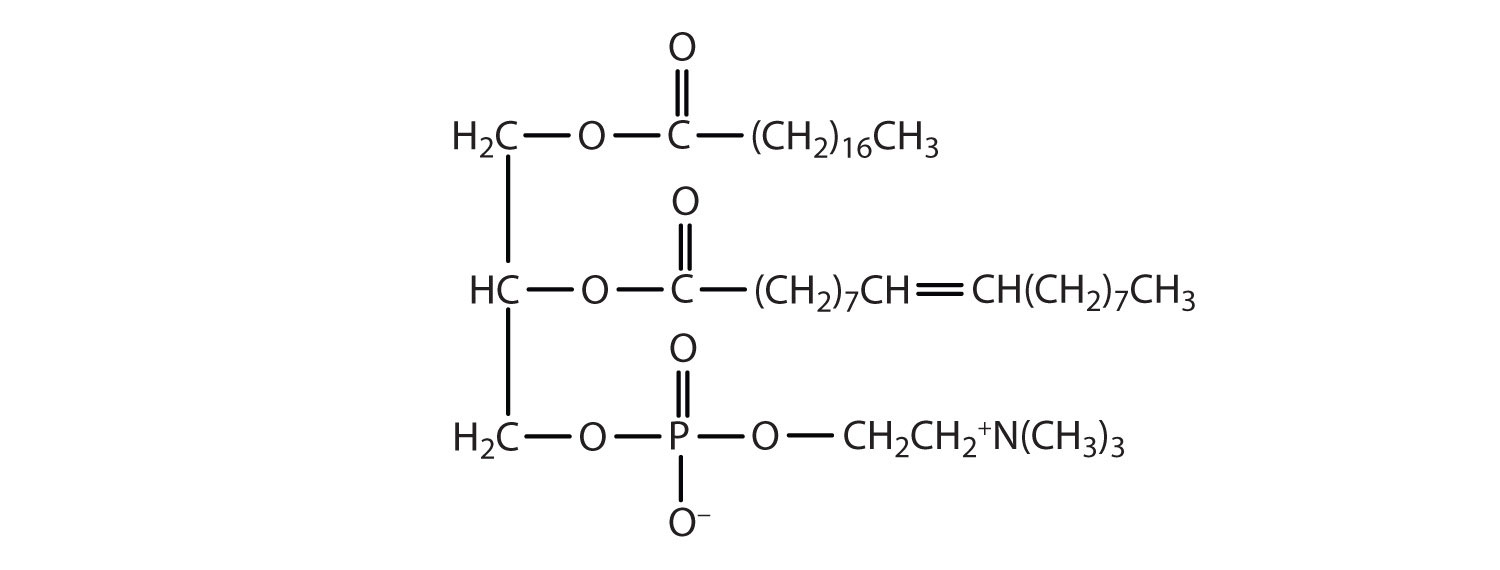
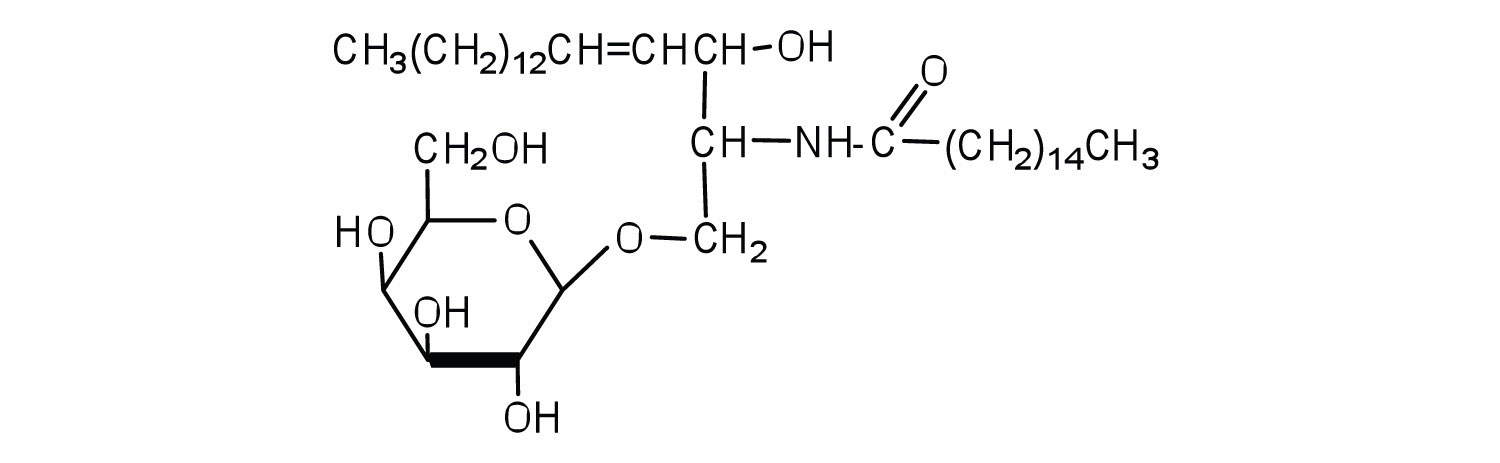
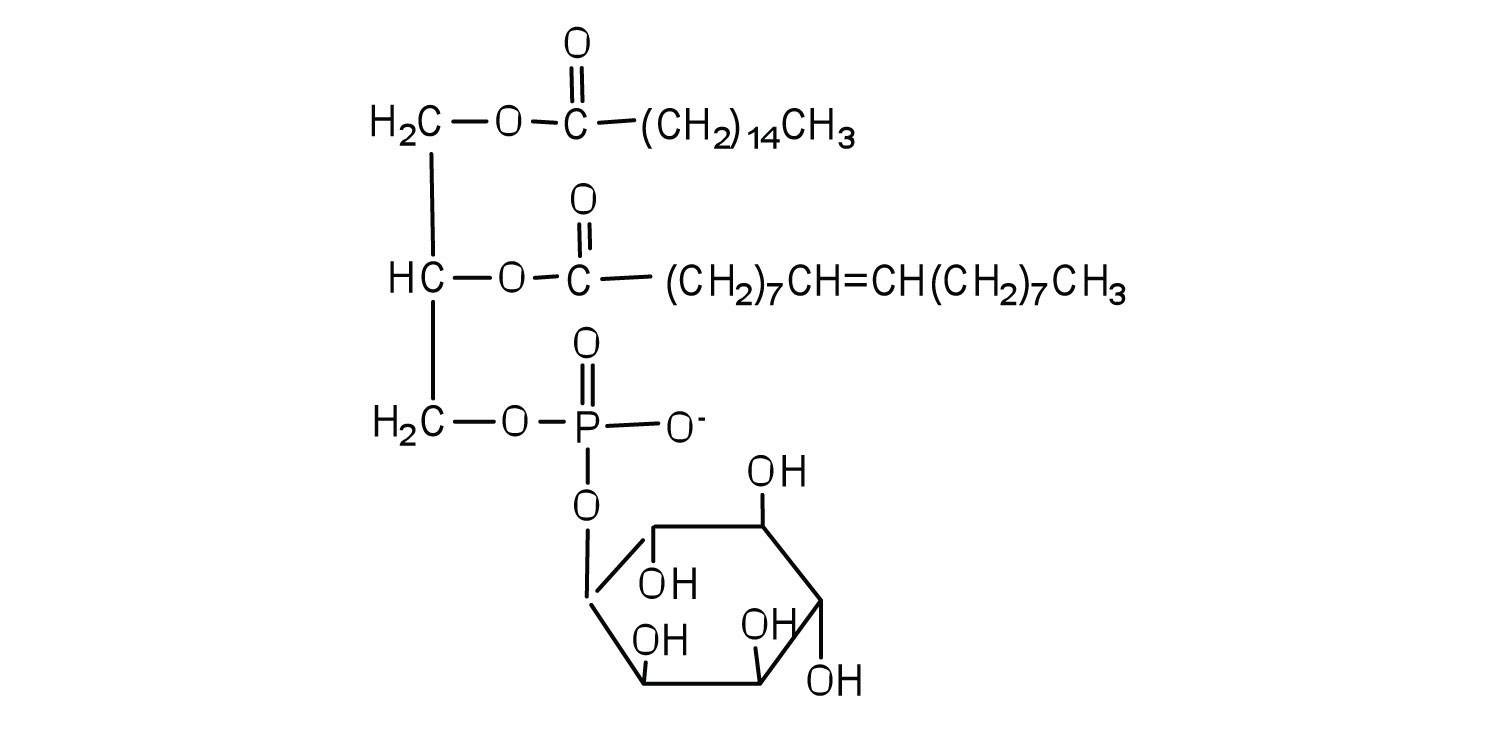
Classify each as a phospholipid, a glycolipid, and/or a sphingolipid. (Some lipids can be given more than one classification.)
-
-
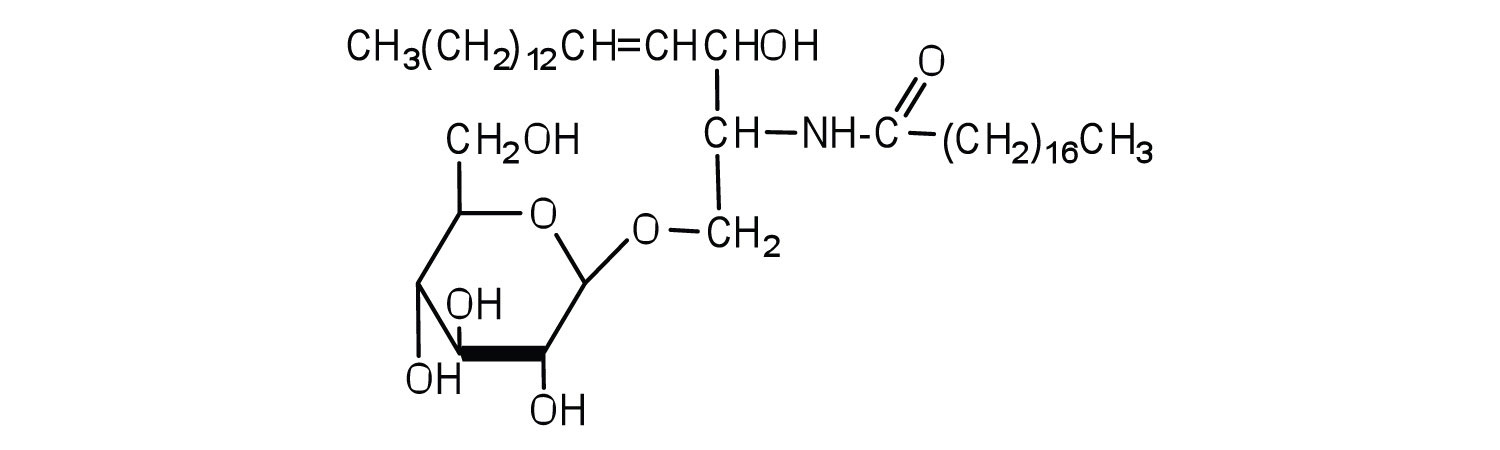
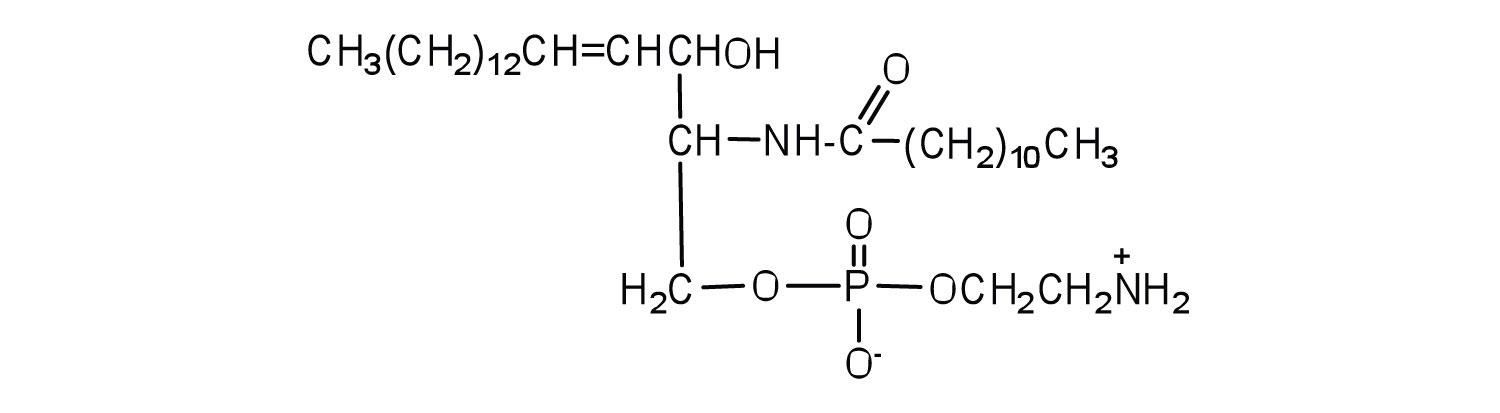
Classify each as a phospholipid, a glycolipid, and/or a sphingolipid. (Some lipids can be given more than one classification.)
-
-
Draw the structure of the sphingomyelin that has lauric acid as its fatty acid and ethanolamine as its amino alcohol.
-
Draw the structure of the cerebroside that has myristic acid as its fatty acid and galactose as its sugar.
-
- Distinguish between an integral protein and a peripheral protein.
- What is one key function of integral proteins?
Answers
-
- phospholipid
- sphingolipid and glycolipid
-
- Integral proteins span the lipid bilayer, while peripheral proteins associate with the surfaces of the lipid bilayer.
- aid in the movement of charged and polar species across the membrane
17.4 Steroids
Concept Review Exercises
-
Distinguish between a saponifiable lipid and a nonsaponifiable lipid.
-
Identify a key function for each steroid.
- bile salt
- cholesterol
- estradiol
Answers
-
A saponifiable lipid reacts with aqueous alkali to yield simpler components, while a nonsaponifiable lipid does not react with alkali to yield simpler components.
-
- acts as an emulsifying agent to break down large fat globules and keep these globules suspended in the aqueous digestive environment
- a key component of mammalian cell membranes (answers will vary)
- stimulates female sex characteristics and regulates changes during the menstrual cycle
Exercises
-
Which of these compounds are steroids—tripalmitin, cephalin, or cholesterol?
-
Which of these compounds are steroids—vitamin D, cholic acid, or lecithin?
-
Draw the basic steroid skeleton and label each ring with the appropriate letter designation.
-
Identify each compound as an adrenocortical hormone, a female sex hormone, or a male sex hormone.
- progesterone
- aldosterone
- testosterone
- cortisol
Answers
-
cholesterol