5.E: Properties of Compounds (Exercises)
- Page ID
- 59369
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)These are homework exercises to accompany Chapter 5 of the University of Kentucky's LibreText for CHE 103 - Chemistry for Allied Health. Solutions are available below the questions.
Questions
(click here for solutions)
Q5.1.1
Define isomer.
Q5.1.2
Circle the chiral carbons in each structure.
| a. | |
| b. | |
| c. | |
| d. | |
| e. | |
| f. | |
| g. | |
| h. | |
| i. | |
| j. |
Q5.1.3
Determine whether each molecule can have a geometric isomer.
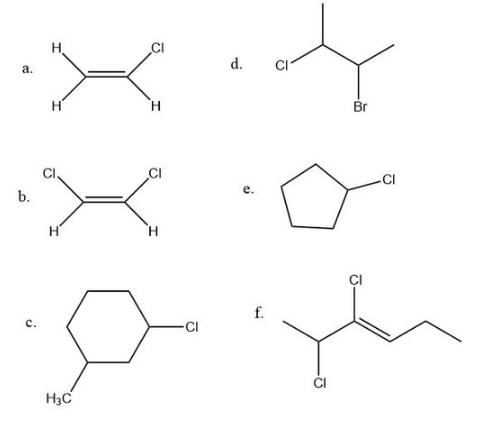
Q5.1.4
For each pair, determine if they are isomers. If they are isomers, identify the type.
| a. |  |
 |
| b. |  |
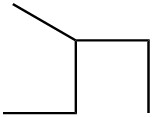 |
| c. | 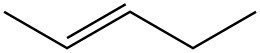 |
 |
| d. | 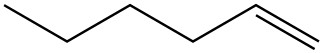 |
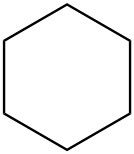 |
| e. | 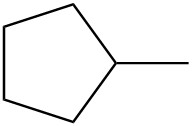 |
 |
| f. |  |
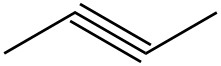 |
| g. | 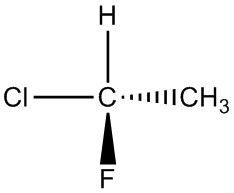 |
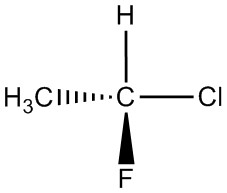 |
| h. | 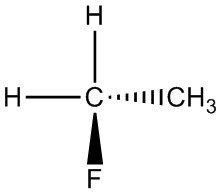 |
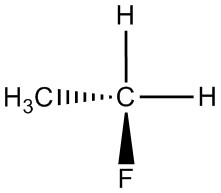 |
Q5.1.5
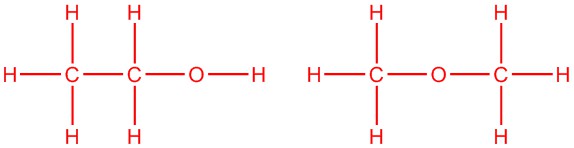
Draw two isomers with the formula C2H6O
(click here for solutions)
Q5.2.1
A monosaccharide has 4 carbon atoms. What is its chemical formula?
Q5.2.2
What are the differences between monosaccharides, disaccharides, oligosaccharides, and polysaccharides?
Q5.2.3
Compare and contrast simple and complex carbohydrates.
Q5.2.4
Draw each of the following structures.
- aldoheptose
- ketopentose
- aldotriose
- ketotetrose
Q5.2.5
Draw the Fischer projections of two aldohexoses that are different from the four given in Figure 5.2.3.
Q5.2.6
Draw the enantiomers of D-glucose, D-allose, D-mannose, and D-galactose. (See Figure 5.2.3).
Q5.2.7
Determine if each of the following pairs are diastereomers? Epimers? (Look up the structures in the chapter or online.)
- D-glucose and D-allose
- D-glucose and D-galactose
- D-allose and D-mannose
- D-allose and D-galactose
Q5.2.8
For each of the given Haworth structures, identify the sugar as being in the α or β form.
| a. | 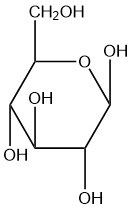 |
| b. | 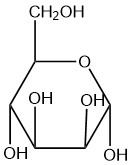 |
| c. | 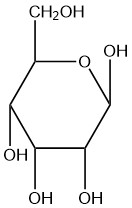 |
| d. | 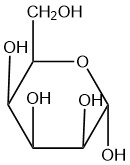 |
| e. | 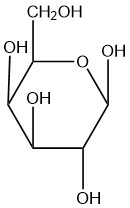 |
Q5.2.9
Refer to Figure 5.2.7 when answering these questions.
- Which blood type is the universal donor? Why?
- Which blood type is the universal acceptor? Why?
- Type A blood can be donated to recipients with which types of blood?
- Type B recipients can accept which types of blood?
Q5.3.1
Define electronegativity.
Q5.3.2
Describe the periodic trends for electronegativity values.
Q5.3.3
Distinguish between nonpolar and polar covalent bonds.
Q5.3.4
Describe the bond between each pair of elements as ionic, polar covalent, or nonpolar covalent. Refer to Table 5.3.1 when answering this question. For the exam, if you need specific values, a table of electronegativity values will be provided. Note that many questions can be answered without the table by knowing the periodic trends.
- N and O
- C and P
- Si and Cl
- Al and F
- Al and I
- P and S
- C and N
- B and Cl
- Be and Br
- Si and P
Q5.3.5
Place the following bonds in order from least to most polar. Refer to Table 5.3.1 when answering this question.
- Fe-N
- H-Cl
- Ca-O
- C-S
Q5.3.6
Place the following bonds in order from least to most polar. Use periodic trends to determine the correct order without looking at electronegativity values.
- PCl
- SCl
- PBr
- CBr
Q5.3.7
Label each of the molecules as nonpolar or polar covalent.
- CO2
- CCl4
- NH3
Q5.3.8
Describe the types of molecules that have the following types of intermolecular forces.
- London dispersion forces
- dipole-dipole forces
- hydrogen bonding
Q5.3.9
Why are the intermolecular forces in H2O and H2S so different from one another?
Q5.3.10
What type(s) of intermolecular forces are present in each of the molecules in the question 5.3.7?
Q5.3.11
What is the relationship between the strength of intermolecular forces in a molecule and its boiling point?
Q5.3.12
Rank the following in order of increasing (smallest to greatest) boiling point.
- N2
- CH3OH
- PH3
Q5.4.1
Define chromatography.
Q5.4.2
List two types of chromatography.
Q5.4.3
Distinguish between the stationary and mobile phases.
Answers
5.1: Isomers
Q5.1.1
Isomers are molecules with the same chemical formula but a different structure or arrangement of atoms.
Q5.1.2
Circle the chiral carbons in each structure.
| a. | 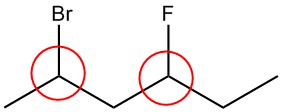 |
| b. | 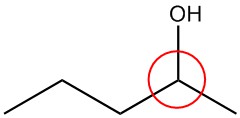 |
| c. | No chiral carbons |
| d. | No chiral carbons |
| e. | 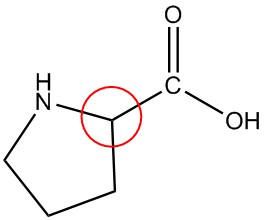 |
| f. | 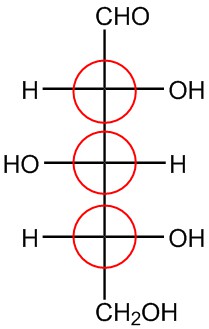 |
| g. | No chiral carbons. |
| h. | 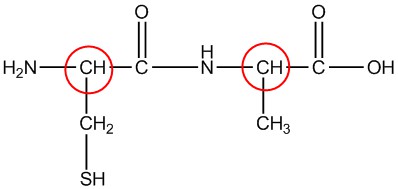 |
| i. | 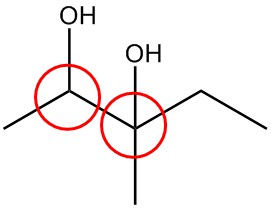 |
| j. |  |
Q5.1.3
- No, because of two H atoms on left side of double bond.
- Yes, because the chlorine atoms are shown trans but could be drawn cis.
- Yes, because the two groups could be on the same side of the ring or on opposite sides.
- No, because you need a double bond or a ring to have a geometric isomer.
- No, because there is only one non-hydrogen group on the ring.
- Yes, because each carbon in the double bond has two different groups so it could be described as cis or trans.
Q5.1.4
For each pair, determine if they are isomers. If they are isomers, identify the type.
- structural
- conformational
- not isomers
- structural
- structural
- not isomers
- enantiomers
- same molecule
Q5.1.5
5.2: Carbohydrate Structures
Q5.2.1
C4H8O4
Q5.2.2
Monosaccharides are a single carbohydrate molecule. Disaccharides have two carbohydrate molecules bonded together. Polysaccharides have three or more carbohydrate molecules bonded together.
Q5.2.3
Simple carbohydrates are monosaccharides or disaccharides and are easily broken down in the body. Complex carbohydrates are polysaccharides such as starches and fiber and take longer to break down in the body.
Q5.2.4
Answers will vary due to orientation of H and OH at each chiral carbon.
| a. | 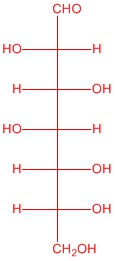 |
| b. | 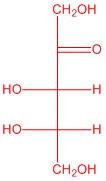 |
| c. | 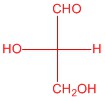 |
| d. | 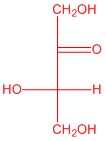 |
Q5.2.5
Any two of the structures shown here in red. The structures in black are from Figure 5.2.3.
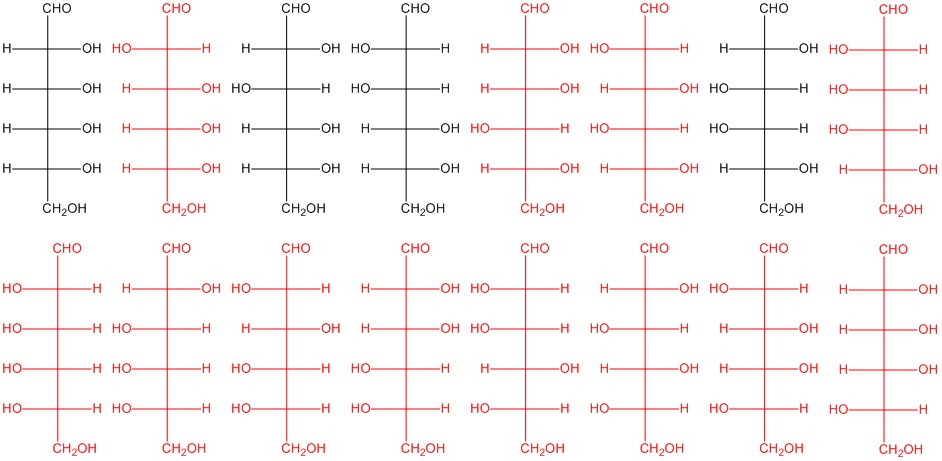
Q5.2.6
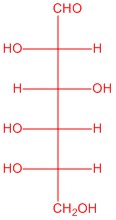 |
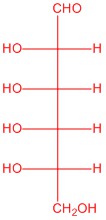 |
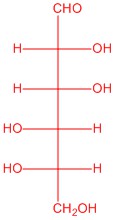 |
 |
| L-glucose | L-allose | L-mannose | L-galactose |
Q5.2.7
- Epimers because the orientation differs at a single chiral carbon.
- Diastereomers because the orientation differs at multiple (but not all) chiral carbons.
- Diastereomers because the orientation differs at multiple (but not all) chiral carbons.
- Diastereomers because the orientation differs at multiple (but not all) chiral carbons.
Q5.2.8
- beta
- alpha
- beta
- alpha
- beta
Q5.2.9
- O because it has the fewest types of carbohydrates.
- AB because it has all carbohydrates found on red blood cells.
- A can give to A or AB.
- B can receive B or O.
5.3: Polarity and Intermolecular Forces
Q5.3.1
Electronegativity is an atom's attraction to an electron in a bond.
Q5.3.2
Electronegativity increases from left to right and from bottom to top with a maximum at fluorine.
Q5.3.3
Nonpolar covalent bonds have even sharing of electrons between atoms while polar electrons share electrons unevenly.
Q5.3.4
- polar
- nonpolar
- polar
- ionic
- polar
- nonpolar
- polar
- polar
- polar
- nonpolar
Q5.3.5
C-S < H-Cl < Fe-N < Ca-O
Calculate the difference in electronegativity for each bond. The smaller the difference, the more nonpolar; the greater the difference, the more polar. If a bond has a large enough difference, it is so polar that it is considered ionic.
Q5.3.6
S-Cl < P-Cl < P-Br < C-Br
- S and Cl are closest together so they will have the smallest difference in electronegativity and be the least polar.
- S-Cl and P-Cl can be compared since they share a common element. P is farther left than S, so the electronegativity of P must be less than that of S. Therefore, the difference between P and Cl must be greater than the difference between S and Cl since we are comparing them both to the same element (Cl).
- Compare P-Cl and P-Br. Since Br is further down the periodic table, it has a lower electronegativity value than Cl. Therefore the difference between P and Cl is less than the difference between P and Br. P-Br is more polar than P-Cl.
- Compare P-Br and C-Br. C is further from Br than P is so the difference in electronegativity is greater for C-Br than P-Br making C-Br the more polar bond.
Q5.3.7
- nonpolar
- nonpolar
- polar
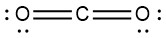 |
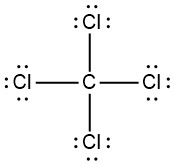 |
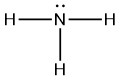 |
Q5.3.8
- all molecules
- polar molecules
- molecules with an H bonded to F, O, or N which is attracted to the F, O, or N on another molecule
Q5.3.9
H2O has hydrogen bonding while H2S does not.
Q5.3.10
- dispersion
- dispersion
- dispersion, dipole-dipole, and hydrogen bonding
Q5.3.11
The stronger the intermolecular forces, the higher the boiling point.
Q5.3.12
N2 < PH3 < CH3OH
All three of these molecules are comparable in size so the differences in dispersion forces are small. N2 is nonpolar and has only dispersion forces. PH3 is polar, so it has dispersion and dipole-dipole forces but no hydrogen bonding. CH3OH has dispersion, dipole-dipole, and hydrogen bonding forces.
Q5.4.1
Chromatogaphy is used to separate a mixture into its components when they travel at different rates in the mobile phase.
Q5.4.2
Paper, thin-layer, liquid, and gas are the most well-known types of chromatography
Q5.4.3
The mobile phase is a fluid which moves through the stationary phase. The stationary phase holds the sample until the mobile phase moves it along the stationary phase.

