22.5: The Liver as a Detox Facility
- Page ID
- 152299
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the different mechanisms of drug detoxification in the liver.
The human liver is thought to be responsible for up to 500 separate functions, usually in combination with other systems and organs. The liver breaks down or modifies toxic substance, such as alcohol and most medicial products, in a process called drug metabolism. This sometimes results in toxication, when the metabolite is more toxic than its precursor.
Alcohol Metabolism
Giving the liver enough time to fully metabolize the ingested alcohol is the only effective way to avoid alcohol toxicity. Drinking coffee or taking a shower will not help. The legal limit for intoxication is a BAC of 0.08. Taking into account the rate at which the liver metabolizes alcohol after drinking stops, and the alcohol excretion rate, it takes at least five hours for a legally intoxicated person to achieve sobriety.
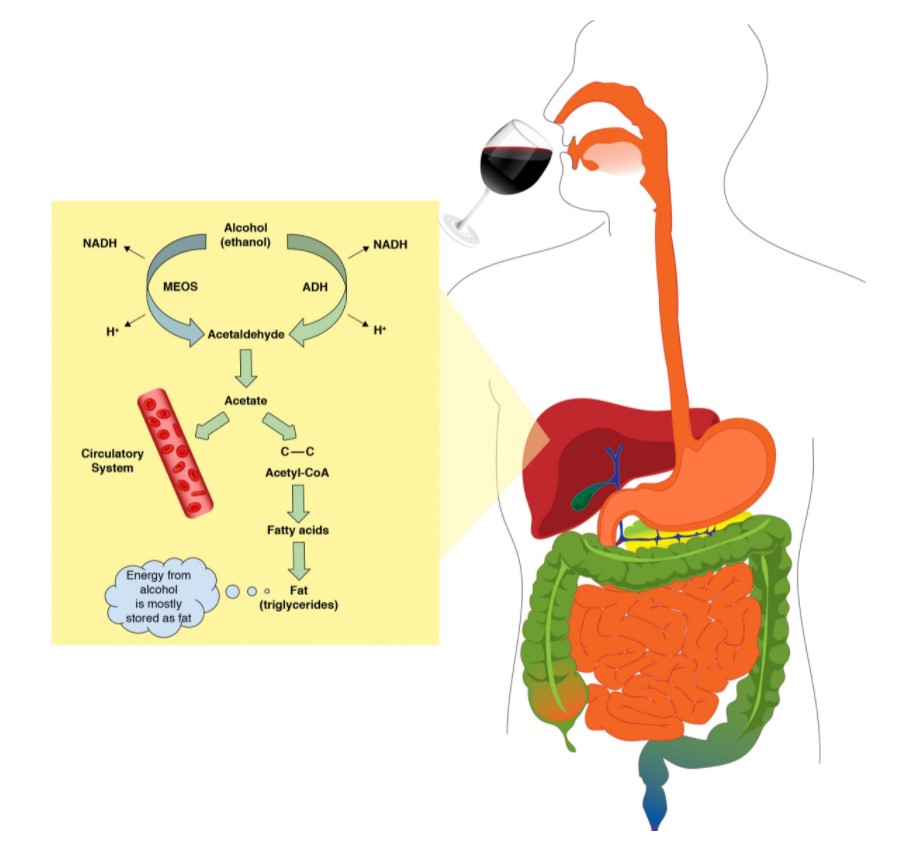
The liver metabolizes up to 85% – 98% of the circulatory ethanol. The liver uses two metabolic processes to get rid of this circulatory ethanol as quickly and safely as possible.
- Alcohol dehydrogenase system
- Microsomal ethanol oxidizing system (MEOS)
Alcohol Dehydrogenase System
About 80 to 90% of the total hepatic ethanol uptake is processed via the alcohol dehydrogenase system.The degradation of ethanol begins in the liver. The enzyme that catalyzes this reaction is called alcohol dehydrogenase. The products from this reaction are acetaldehyde, NADH (a reduced coenzyme that carries electrons from one reaction to another) and H+ ion. Acetaldehyde is very toxic to the liver and the body’s cells. The moment acetaldehyde is produced; it must be degraded to protect the liver cells. The enzyme that will carry this type of degradation reaction is acetaldehyde dehydrogenase (ALDH). Acetaldehyde dehydrogenase converts acetaldehyde into acetate, a non-toxic molecule.
Microsomal Ethanol Oxidizing System (MEOS)
In a moderate drinker, about 10 to 20% of the total liver ethanol uptake is processed via the microsomal ethanol oxidizing system (MEOS). During periods of heavy drinking, the MEOS system will metabolize most of the excess ethanol ingested. Heavy drinking stimulates the human body to include the MEOS system enzymes to clear ethanol faster from the body.
The MEOS system is also located in the liver. Similar to the Alcohol dehydrogenase system, acetaldehyde dehydrogenase will immediately convert acetaldehyde into acetate, a non-toxic molecule. Other products from this reaction are NADH and H+ ion.
Fate of Acetate
The acetate produced (from the alcohol dehydrogenase system and microsomal ethanol oxidizing system) is either released into circulation or retained inside the liver cells. In the liver cells, acetate is converted to acetyl CoA where it is used to produce other molecules like CO2 or used in the synthesis of fatty acids and cholesterol.
Nicotine and Ammonia
In humans, nicotine is extensively metabolized in the liver into various metabolites. About 70-80% of nicotine is broken down to cotinine, the predominant metabolite. Other primary metabolites include nicotine N'-oxide, nornicotine, nicotine isomethonium ion, 2-hydroxynicotine and nicotine glucuronide. Under some conditions, other substances may be formed such as myosmine.

The liver converts ammonia into urea as part of the ornithine cycle or the urea cycle, and the urea is excreted in the urine.
Summary
- The liver is involved in the breakdown or modification of various substances in a process called drug metabolism, but the metabolites are not directly removed from the body.
- Ethanol is broken down through a series of steps (involving various liver enzymes) to carbon dioxide and water.
- Ethanol therapy is the traditional treatment for methanol and ethylene glycol poisoning.
- Nicotine is extensively metabolized in the liver into a variety of breakdown products whereas toxic ammonia is converted to non toxic urea.
Contributors and Attributions
- Wikipedia
- Benowitz, N. L., Hujjanen, J., Peyton III, J. Nicotine Chemistry, Metabolism, Kinetics, and Biomarkers. Hand Exp Pharmacol. 2009. (192): 29-60.
- Template:ContribUofHawaiiNutrition

