21.6: Cosmetics - Personal Care Chemicals
- Page ID
- 152267
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- List the main ingredients in different personal care products and their functions.
- Describe the steps in formation of temporary and permanent waves.
In the United States, the Food and Drug Administration (FDA), which regulates cosmetics, defines cosmetics as products "intended to be applied to the human body for cleansing, beautifying, promoting attractiveness, or altering the appearance without affecting the body's structure or functions". This broad definition includes any material intended for use as an ingredient of a cosmetic product, with the FDA specifically excluding pure soap from this category.

Cosmetics (Figure \(\PageIndex{1}\)) have been in use for thousands of years. Egyptian men and women used makeup to enhance their appearance. They were very fond of eyeliner and eye-shadows in dark colors including blue, red, and black. Ancient Sumerian men and women were possibly the first to invent and wear lipstick, about 5,000 years ago. They crushed gemstones and used them to decorate their faces, mainly on the lips and around the eyes.
According to one source, early major developments include:
- Kohl used by ancient Egypt as a protectant of the eye (Figure \(\PageIndex{2}\)).
- Castor oil used by ancient Egypt as a protective balm.
- Skin creams made of beeswax, olive oil, and rose water, described by Romans.
- Vaseline and lanolin in the nineteenth century.
Although modern makeup has been traditionally used mainly by women, an increasing number of men are using cosmetics usually associated with women to enhance or cover their own facial features such as blemishes and dark circles, as well the use of eyeshadow, mascara and lipstick by some. Cosmetics brands have increasingly also targeted men in the sale of cosmetics, with some products targeted specifically at men.

The Skin
The cutaneous membrane is the technical term for our skin. The skin’s primary role is to help protect the rest of the body’s tissues and organs from physical damage such as abrasions, chemical damage such as detergents, and biological damage from microorganisms. For example, while the skin harbors many permanent and transient bacteria, these bacteria are unable to enter the body when healthy, intact skin is present.
Our skin is made of three general layers (Figure \(\PageIndex{3}\)). In order from most superficial to deepest they are the epidermis, dermis, and subcutaneous tissue (hypodermis).
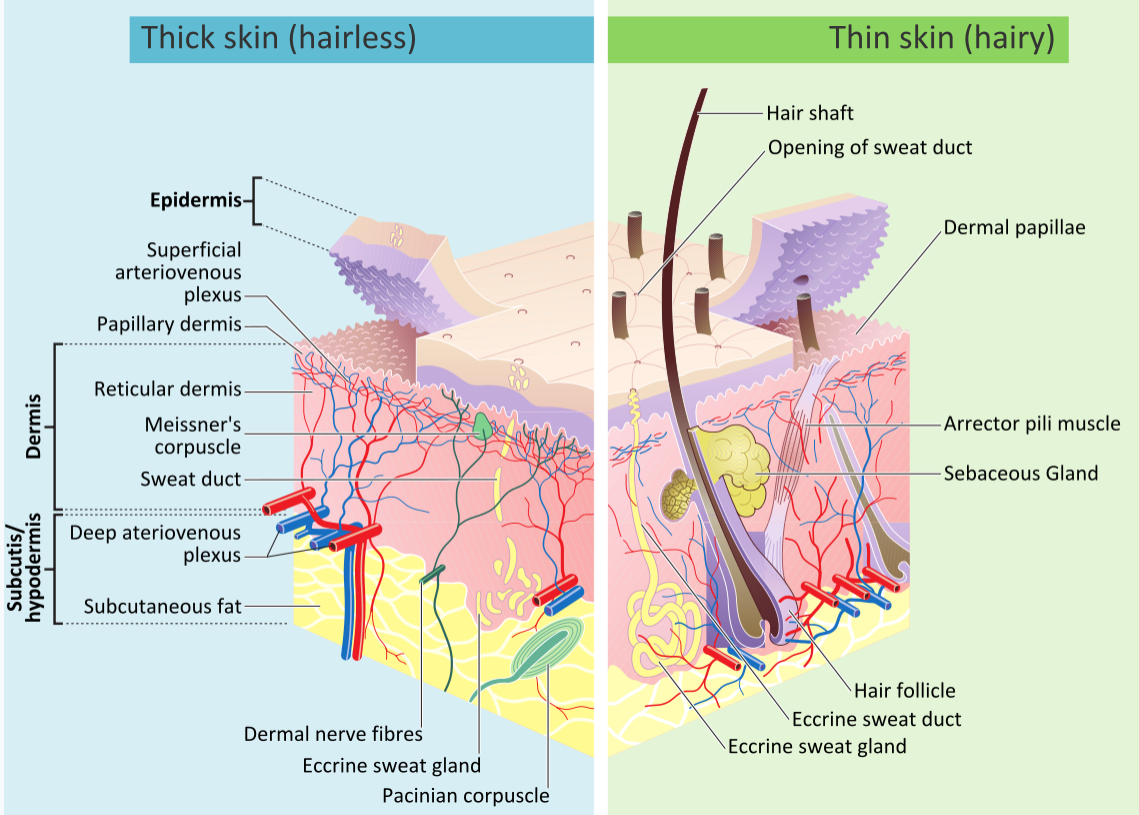
There are several different types of cells in the epidermis. All of the cells are necessary for the important functions of the epidermis.
- The epidermis consists mainly of stacks of keratin-producing epithelial cells called keratinocytes. These cells make up at least 90 percent of the epidermis. Near the top of the epidermis, these cells are also called squamous cells.
- Another 8 percent of epidermal cells are melanocytes. These cells produce the pigment melanin that protects the dermis from UV light.
- About 1 percent of epidermal cells are Langerhans cells. These are immune system cells that detect and fight pathogens entering the skin.
- Less than 1 percent of epidermal cells are Merkel cells, which respond to light touch and connect to nerve endings in the dermis.
The epidermis has several crucial functions in the body. These functions include protection, water retention, and vitamin D synthesis. The epidermis provides protection to underlying tissues from physical damage, pathogens, and UV light.
Skin Creams and Lotions
A lotion is a low-viscosity topical preparation intended for application to the skin. By contrast, creams and gels have higher viscosity, typically due to lower water content. Lotions are applied to external skin with bare hands, a brush, a clean cloth, or cotton wool.
While a lotion may be used as a medicine delivery system, many lotions, especially hand lotions and body lotions are meant instead to simply smooth, moisturize, soften and perhaps perfume the skin.
Most cosmetic lotions are moisturizing lotions, although other forms, such as tanning lotion, also exist.
Cosmetic lotions may be marketed as anti-aging lotions, which can also be classified as a cosmetic in many cases, and may contain fragrances. The Food and Drug Administration voiced concern about lotions not classified as drugs that advertise anti-aging or anti-wrinkle properties.
Most lotions are oil-in-water emulsions using a substance such as cetearyl alcohol to keep the emulsion together, but water-in-oil lotions are also formulated.
A cream is a preparation usually for application to the skin. Creams for application to mucous membranes such as those of the rectum or vagina are also used. Creams may be considered pharmaceutical products as even cosmetic creams are based on techniques developed by pharmacy and unmedicated creams are highly used in a variety of skin conditions (dermatoses). The use of the finger tip unit concept may be helpful in guiding how much topical cream is required to cover different areas.
Creams are semi-solid emulsions of oil and water. They are divided into two types: oil-in-water (O/W) creams which are composed of small droplets of oil dispersed in a continuous water phase, and water-in-oil (W/O) creams which are composed of small droplets of water dispersed in a continuous oily phase. Oil-in-water creams are more comfortable and cosmetically acceptable as they are less greasy and more easily washed off using water. Water-in-oil creams are more difficult to handle but many drugs which are incorporated into creams are hydrophobic and will be released more readily from a water-in-oil cream than an oil-in-water cream. Water-in-oil creams are also more moisturizing as they provide an oily barrier which reduces water loss from the stratum corneum, the outermost layer of the skin.
Ointment is a semisolid dosage form it is used for topical application to the medication
Water, oil, emulsifier, and thickening agent are four main ingredients of cold creams and lotions.
Moisturizer or moisturiser is a cosmetic preparation used for protecting, moisturizing, and lubricating the skin. These functions are normally performed by sebum produced by healthy skin.
Moisturizers modify the rate of water loss, with active ingredients of moisturizers falling into one of two categories: occlusives and humectants
Occlusives form a coating on the surface of the skin, keeping moisture from escaping.
Humectants absorb water. They can absorb this water from the air and moisturize the skin when the humidity is greater than 70%, but more commonly they draw water from the dermis into the epidermis, making skin dryer. A study published in Skin Research and Technology in 2001 found no link between humectants and moisturizing effect. When used in practical applications, they are almost always combined with occlusives.
Moisturizers often contain water, which acts as a temporary hydration agent and as a way for the absorption of some components and evaporation of the moisturizer.
There are many different types of moisturizers. Petrolatum is one of the most effective moisturizers, although it can be unpopular due to its oily consistency. Other popular moisturizers are cetyl alcohol, cetearyl alcohol, cocoa butter, isopropyl myristate, isopropyl palmitate, lanolin, liquid paraffin, polyethylene glycols, shea butter, silicone oils, stearic acid, stearyl alcohol and castor oil, as well as other oils.
Moisturizers may also be available as lotions, creams, ointments, bath oils, or soap substitutes.
Mineral oils and waxes are insensitive to oxidation or rancidity. For this reason, they have essentially replaced vegetable oils in emollients and topical medication.
Moisturizer cosmetics may additionally contain antioxidants, ceramides, emulsifiers, fragrances, penetration enhancers, preservatives, and solvents. Some products are marketed as having anti-wrinkle and skin enhancement effects. Many plant and animal extracts have been claimed to impart skin benefits, with little scientific evidence.
Shaving Creams
Shaving cream or shave cream is a category of cosmetics used for shaving preparation. The purpose of shaving cream is to soften the hair by providing lubrication.
Different types of shaving creams include aerosol shaving cream (also known as shaving foam), latherless shaving cream (also called brushless shaving cream and non-aerosol shaving cream), and lather shaving cream or lathering shaving cream. The term shaving cream can also refer to the lather produced with a shaving brush from shaving soap or a lather shaving cream.
Shaving creams commonly consist of an emulsion of oils, soaps or surfactants (e.g. triethanolamine steatrate), and water. In addition to soap, lather shaving creams include a humectant for softer consistency and keeping the lather moisturised. Brushless shaving creams, on the other hand, don't contain soap and so don't produce lather. They are an oil-in-water mixture to which humectants, wetting agents, and other ingredients are added. Aerosol shaving creams are basically lather shaving cream in liquid form with propellants, vegetable waxes, and various oils added.
Video \(\PageIndex{1}\) What is shaving cream?
Sunscreen and Sunblock
UVB radiation in sunlight allows the skin to produce vitamin D. This vitamin prevents bone disorders like rickets and osteoporosis (brittle bone disease). The American Academy of Dermatology suggests vitamin D be obtained through foods or nutritional supplements. Excessive exposure to UV can be damaging and the pigment melanin, deposited in cells at the base of the epidermis, helps to protect the underlying layers of the skin from this damage. Melanin also colors the skin and variations in the amount of melanin produces colors from pale yellow to black. The darker the skin tone, the more melanin one has, and the less likely skin cancer will occur.
Excess exposure to the sun can cause sunburn. This is common in humans, but light skinned animals like cats and pigs can also be sunburned, especially on the ears. Skin cancer can also result from excessive exposure to the sun. As holes in the ozone layer increase exposure to the sun’s UV rays, so too does the rate of skin cancer in humans and animals.
Sunscreens and sunblocks are designed to protect skin from ultraviolet rays. Sunblocks contain inorganic ingredients like zinc oxide or titanium dioxide. These chemicals act as UV filters by reflecting the sun's UV rays. Sunblocks can have grainy textures due to the inorganic components. The thick nature of a sunblock can make it difficult to spread evenly on the skin. Sunscreens contain organic compounds like oxybenzone, avobenzone, homosalate, and octinoxate (Figure \(\PageIndex{4}\)). By absorbing ultraviolet rays, these compounds decompose and give off heat. Sunscreens apply smoother than sunblocks. Often, manufacturers will combine sunscreen and sunblock ingredients to make their products.
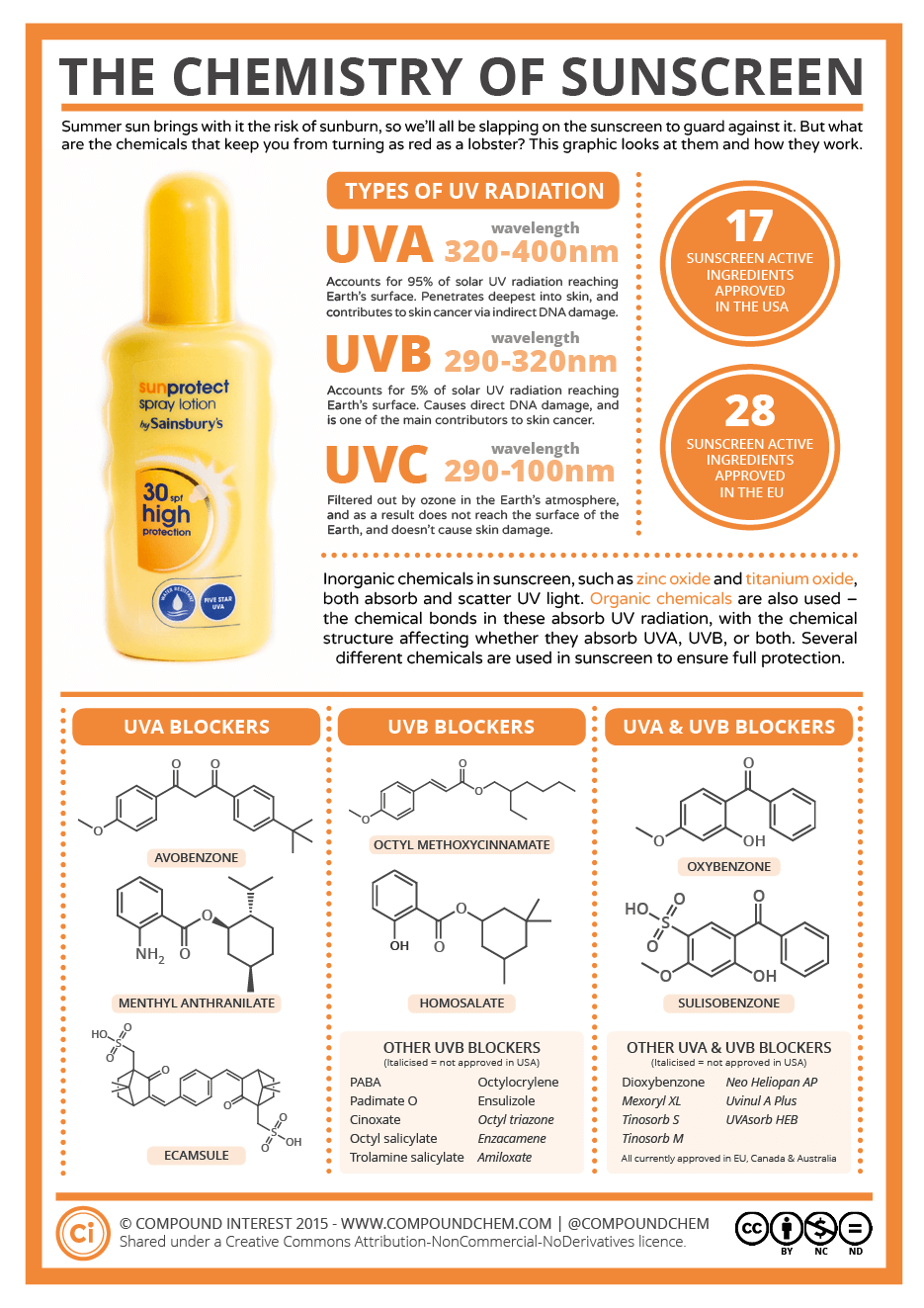
Sun protection factor (SPF) measures a product's protection from UVB rays. SPF does not quantify protection from UVA radiation. The American Academy of Dermatology recommends you select a sunscreen or sunblock with a minimum SPF factor of 30. This SPF value means a lotion can filter out 97% of UVB rays. Moving to a SPF of 50 will only filter out 1 more percentage of UVB rays. Increasing a SPF factor past 30 really does very little in shielding skin from UVB radiation.
Products that protect skin from UVA must be labeled as being a broad spectrum. Sunblocks provide UVA and UVB protection, but sunscreens can vary on what they can screen. Every two hours, sunscreens/sunblocks should be reapplied to the skin. Sweating and swimming can remove sunscreen/sunblock products. No sun products are waterproof, but some are labeled as being water-resistant. Consumers are encouraged to reapply these products every two hours as well.
Figure \(\PageIndex{5}\) Sunscreen label. Source: Badger: Healthy Body Care
Check the link below on FAQs regarding sunscreen.
https://www.aad.org/public/everyday-care/sun-protection/sunscreen/understand-sunscreen-labels
Lipsticks and Lip Balms
Lipstick, lip gloss, lip liner, lip plumper, lip balm, lip stain, lip conditioner, lip primer, lip boosters, and lip butters: Lipsticks are intended to add color and texture to the lips and often come in a wide range of colors, as well as finishes such as matte, satin, gloss and lustre.
Lipstick contains wax, oils, antioxidants, and emollients. Wax provides the structure to the solid lipstick. Lipsticks may be made from several waxes such as beeswax, ozokerite and candelilla wax. Because of its high melting point, carnauba wax is a key ingredient in terms of strengthening the lipstick. Various oils and fats are used in lipsticks, such as olive oil, mineral oil, cocoa butter, lanolin, and petrolatum.
Lipsticks get their colors from a variety of pigments and lake dyes including, but not limited to bromo acid, D&C Red No. 21, Calcium Lake such as D&C Red 7 and D&C Red 34, and D&C Orange No. 17. Pink lipsticks are made by mixing white titanium dioxide and red shades. Both organic and inorganic pigments are employed.
Lip stains have a water or gel base and may contain alcohol to help the product stay on leaving a matte look. They temporarily saturate the lips with a dye. Usually designed to be waterproof, the product may come with an applicator brush, directly through the applicator, rollerball, or could be applied with a finger. Lip glosses are intended to add shine to the lips and may add a tint of color, as well as being scented or flavored. Lip balms are most often used to moisturize, tint, and protect the lips. Some brands contain sunscreen. Using a priming lip product such as lip balm or chapstick can prevent chapped lips.
Eye Make-up
- Mascara is a cosmetic commonly used to enhance the eyelashes. It may darken, thicken, lengthen, and/or define the eyelashes. Normally in one of three forms—liquid, powder, or cream—the modern mascara product has various formulas; however, most contain the same basic components of pigments, oils, waxes, and preservatives. The most common form of mascara is a liquid in a tube.
- Eye shadow (or eyeshadow) is a cosmetic applied primarily to the eyelids to make the wearer's eyes stand out or look more attractive. Eye shadow can also be applied under eyes or to brow bones.
Eye shadow can add depth and dimension to one's eyes, complement one's eye color, make one's eyes appear larger, or simply draw attention to the eyes. Eye shadow comes in many different colors and textures. It is usually made from a powder but can also be found in liquid, pencil, cream or mousse form. Just like other trends, eyeshadow looks also have trends.
Eye shadows typically consist of four types of ingredients: base fillers, binders, slip and preservatives. In order to make eye shadow, there must be a balance between the fillers and binders.
Base fillers are usually minerals such as mica, talc or kaolin clay, which add bulk and texture to eye shadow. They make up about 30% of eye shadow powders and 25% of cream eye shadows. Mica absorbs moisture, gives the eye shadow shine and luster, and makes it opaque. Mica powders, iron oxides and clays can give color pigments to eye shadows.
Binders help eye shadow adhere and stay attached to skin. Eye shadows can have dry or liquid binders. Zinc and magnesium, which are both white powders, are commonly used as dry binders. Zinc also adds color and can be used to increase the thickness of the eyeshadow. Silicone, paraffin wax, mineral oil or vegetable oils may be used as liquid binders.
Slip allows eye shadow to glide across the skin smoothly. Products may use silica or nylon, which are fine, colorless powders. Other types of slip include dimethicone, boron nitride or bismuth oxychloride.
Preservatives help products stay bacteria free and extend their lifespan. Common preservatives in eye shadow are glycol and tocopherol.

Eye liner or eyeliner is a cosmetic used to define the eyes. It is applied around the contours of the eye(s) to create a variety of aesthetic effects. It can come in the form of a pencil, a gel, or a liquid and can be found in almost any color. Traditional wax-based eye liners are made from about 20 components. About 50% by weight are waxes (e.g., Japan wax, fats, or related soft materials that easily glide on to the skin. Stearyl heptanoate is found in most cosmetic eyeliner. Typical pigments include black iron oxides, as well as smaller amounts of titanium dioxide and Prussian blue.
Eyebrow shaders, and pencils are often used to define the eyebrow or make it appear fuller. These can create an outline for the brows or mimic hairs where there are sparse areas. Brow gels are also used in creating a thicker brow; they allow for the hairs to be more textured, which gives the appearance of thicker, fuller brows. Lastly, brow powders or even eye shadows are used for those who want a fuller and more natural look, by placing the brow powder or eye shadow (closest to the natural hair color) in areas where there is less hair.
Deodorants and Antiperspirants
A deodorant is a substance applied to the body to prevent or mask body odor due to bacterial breakdown of perspiration in the armpits, groin, and feet, and in some cases vaginal secretions. A subclass of deodorants, called antiperspirants, prevents sweating itself, typically by blocking sweat glands. Antiperspirants are used on a wider range of body parts, at any place where sweat would be inconvenient or unsafe, since unwanted sweating can interfere with comfort, vision, and grip (due to slipping). Other types of deodorant allow sweating but prevent bacterial action on sweat, since human sweat only has a noticeable smell when it is decomposed by bacteria.
In the United States, the Food and Drug Administration classifies and regulates most deodorants as cosmetics but classifies antiperspirants as over-the-counter drugs.
In the United States, deodorants are classified and regulated as cosmetics by the U.S. Food and Drug Administration (FDA) and are designed to eliminate odor. Deodorants are often alcohol based. Alcohol initially stimulates sweating but may also temporarily kill bacteria. Other active ingredients in deodorants include sodium stearate, sodium chloride, and stearyl alcohol. Deodorants can be formulated with other, more persistent antimicrobials such as triclosan that slow bacterial growth or with metal chelant compounds such as EDTA. Deodorants may contain perfume fragrances or natural essential oils intended to mask the odor of perspiration. In the past, deodorants included chemicals such as zinc oxide, acids, ammonium chloride, sodium bicarbonate, and formaldehyde, but some of these ingredients were messy, irritating to the skin, or even carcinogenic.
Over-the-counter products, often labeled as "natural deodorant crystal", contain the chemical rock crystals potassium alum or ammonium alum, which prevents bacterial action on sweat. These have gained popularity as an alternative health product, in spite of concerns about possible risks related to aluminum (see below – all alum salts contain aluminum in the form of aluminum sulphate salts) and contact dermatitis.
In the United States, deodorants combined with antiperspirant agents are classified as drugs by the FDA. Antiperspirants attempt to stop or significantly reduce perspiration and thus reduce the moist climate in which bacteria thrive. Aluminium chloride, aluminium chlorohydrate, and aluminium-zirconium compounds, most notably aluminium zirconium tetrachlorohydrex gly and aluminium zirconium trichlorohydrex gly, are frequently used in antiperspirants. Aluminium chlorohydrate and aluminium-zirconium tetrachlorohydrate gly are the most frequent active ingredients in commercial antiperspirants. Aluminium-based complexes react with the electrolytes in the sweat to form a gel plug in the duct of the sweat gland. The plugs prevent the gland from excreting liquid and are removed over time by the natural sloughing of the skin. The metal salts work in another way to prevent sweat from reaching the surface of the skin: the aluminium salts interact with the keratin fibrils in the sweat ducts and form a physical plug that prevents sweat from reaching the skin’s surface. Aluminium salts also have a slight astringent effect on the pores; causing them to contract, further preventing sweat from reaching the surface of the skin. The blockage of a large number of sweat glands reduces the amount of sweat produced in the underarms, though this may vary from person to person. Methenamine (Figure \(\PageIndex{7}\)) in the form of cream or spray is effective in the treatment of excessive sweating and attendant odor. Antiperspirants are usually best applied before bed.

Toothpaste: Soap with Grit and Flavor
Toothpaste (Figure \(\PageIndex{8}\)) is a paste or gel dentifrice used with a toothbrush to clean and maintain the aesthetics and health of teeth. Toothpaste is used to promote oral hygiene: it is an abrasive that aids in removing dental plaque and food from the teeth, assists in suppressing halitosis, and delivers active ingredients (most commonly fluoride) to help prevent tooth decay (dental caries) and gum disease (gingivitis). Salt and sodium bicarbonate (baking soda) are among materials that can be substituted for commercial toothpaste. Large amounts of swallowed toothpaste can be toxic.

In addition to 20%–42% water, toothpastes are derived from a variety of components, the three main ones being abrasives, fluoride, and detergents.
Abrasives constitute at least 50% of a typical toothpaste. These insoluble particles are designed to help remove plaque from the teeth. The removal of plaque and calculus prevents the accumulation of tartar and is widely claimed to help minimize cavities and periodontal disease, although the clinical significance of this benefit is debated. Representative abrasives include particles of aluminum hydroxide (Al(OH)3), calcium carbonate (CaCO3), various calcium hydrogen phosphates, various silicas and zeolites, and hydroxyapatite (Ca5(PO4)3OH).
Abrasives, like the dental polishing agents used in dentists' offices, also cause a small amount of enamel erosion which is termed "polishing" action. Some brands contain powdered white mica, which acts as a mild abrasive, and also adds a cosmetically pleasing glittery shimmer to the paste. The polishing of teeth removes stains from tooth surfaces, but has not been shown to improve dental health over and above the effects of the removal of plaque and calculus.
The abrasive effect of toothpaste is indicated by its RDA value. Too high RDA values are deleterious. Some dentists recommend toothpaste with an RDA value no higher than 50 for daily use.
Fluoride in various forms is the most popular active ingredient in toothpaste to prevent cavities. Fluoride is present in small amounts in plants, animals, and some natural water sources. The additional fluoride in toothpaste has beneficial effects on the formation of dental enamel and bones. Sodium fluoride (NaF) is the most common source of fluoride, but stannous fluoride (SnF2), olaflur (an organic salt of fluoride), and sodium monofluorophosphate (Na2PO3F) are also used. Stannous fluoride has been shown to be more effective than sodium fluoride in reducing the incidence of dental caries and controlling gingivitis, but causes somewhat more surface stains.
Much of the toothpaste sold in the United States has 1,000 to 1,100 parts per million fluoride. In European countries, such as the UK or Greece, the fluoride content is often higher; a NaF content of 0.312% w/w (1,450 ppm fluoride) is common. All of these concentrations are likely to prevent tooth decay, according to a 2019 Cochrane review. Concentrations below 1,000 ppm are not likely to be preventive, and the preventive effect increases with concentration. Clinical trials support the use of high fluoride dentifrices, as it was found to reduce the amount of plaque accumulated, decrease the number of mutans streptococci and lactobacilli and possibly promote calcium fluoride deposits to a higher degree than after the use of traditional fluoride containing dentifrices. However, these effects must be balanced with the increased risk of harm at higher concentrations.
Many, although not all, toothpastes contain sodium lauryl sulfate (SLS) or related surfactants (detergents). SLS is found in many other personal care products as well, such as shampoo, and is mainly a foaming agent, which enables uniform distribution of toothpaste, improving its cleansing power.
Other Components in toothpaste formulations include:
- Antibacterial agents. Triclosan or zinc chloride prevent gingivitis and, according to the American Dental Association, helps reduce tartar and bad breath.
- Flavorants. Toothpaste comes in a variety of colors and flavors intended to encourage use of the product. The three most common flavorants are peppermint, spearmint, and wintergreen.
- Reminalizers. Hydroxyapatite nanocrystals and a variety of calcium phosphates are included in formulations for remineralization, i.e. the reformation of enamel.
Agents are added to suppress the tendency of toothpaste to dry into a powder. Included are various sugar alcohols, such as glycerol, sorbitol, or xylitol, or related derivatives, such as 1,2-propylene glycol and polyethyleneglycol
Strontium chloride or potassium nitrate is included in some toothpastes to reduce sensitivity. Two systemic meta-analysis reviews reported that arginine, and calcium sodium phosphosilicate - CSPS containing toothpastes are also effective in alleviating dentinal hypersensitivity respectively. Another randomized clinical trial found superior effects when both formulas were combined together.
Sodium polyphosphate is added to minimize the formation of tartar. Other example to components in toothpastes is the Biotene, which has proved its efficiency in relieving the symptoms of dry mouth in people who suffer from xerostomia according to the results of two randomized clinical trials.
Chlorohexidine mouthwash has been popular for its positive effect on controlling plaque and gingivitis, however, a systemic review studied the effects of chlorohexidine toothpastes and found insufficient evidence to support its use, tooth surface discoloration was observed as a side effect upon using it, which is considered a negative side effect that can affect patients' compliance.
Sodium hydroxide, also known as lye or caustic soda, is listed as an inactive ingredient in some toothpaste, for example Colgate Total.
Some studies have demonstrated that toothpastes with xylitol as an ingredient are more effective at preventing dental caries in permanent teeth of children than toothpastes containing fluoride alone.
Perfumes, Colognes, and Aftershaves
Perfume (Figure \(\PageIndex{9}\)) is a mixture of fragrant essential oils or aroma compounds, fixatives and solvents, used to give the human body, animals, food, objects, and living-spaces an agreeable scent. Perfume types reflect the concentration of aromatic compounds in a solvent, which in fine fragrance is typically ethanol or a mix of water and ethanol. Various sources differ considerably in the definitions of perfume types. The intensity and longevity of a perfume is based on the concentration, intensity, and longevity of the aromatic compounds, or perfume oils, used. Specific terms are used to describe a fragrance's approximate concentration by the percent of perfume oil in the volume of the final product. The most widespread terms are:
- parfum or extrait, in English known as perfume extract, pure perfume, or simply perfume: 15–40% aromatic compounds (IFRA: typically ~20%);
- esprit de parfum (ESdP): 15–30% aromatic compounds, a seldom used strength concentration in between EdP and perfume;
- eau de parfum (EdP) or parfum de toilette (PdT) (The strength usually sold as "perfume"): 10–20% aromatic compounds (typically ~15%); sometimes called "eau de perfume" or "millésime"; parfum de toilette is a less common term, most popular in the 1980s, that is generally analogous to eau de parfum;
- eau de toilette (EdT): 5–15% aromatic compounds (typically ~10%); This is the staple for most masculine perfumes.
- eau de Cologne (EdC): often simply called cologne: 3–8% aromatic compounds (typically ~5%);
- eau fraiche: products sold as "splashes", "mists", "veils" and other imprecise terms. Generally these products contain 3% or less aromatic compounds and are diluted with water rather than oil or alcohol.

Perfume is described in a musical metaphor as having three sets of notes, making the harmonious scent accord. The notes unfold over time, with the immediate impression of the top note leading to the deeper middle notes, and the base notes gradually appearing as the final stage. These notes are created carefully with knowledge of the evaporation process of the perfume.
- Top notes: Also called the head notes. The scents that are perceived immediately on application of a perfume. Top notes consist of small, light molecules that evaporate quickly. They form a person's initial impression of a perfume and thus are very important in the selling of a perfume. Examples of top notes include mint, lavender and coriander.
- Middle notes: Also referred to as heart notes. The scent of a perfume that emerges just prior to the dissipation of the top note. The middle note compounds form the "heart" or main body of a perfume and act to mask the often unpleasant initial impression of base notes, which become more pleasant with time. Examples of middle notes include seawater, sandalwood and jasmine.
- Base notes: The scent of a perfume that appears close to the departure of the middle notes. The base and middle notes together are the main theme of a perfume. Base notes bring depth and solidity to a perfume. Compounds of this class of scents are typically rich and "deep" and are usually not perceived until 30 minutes after application. Examples of base notes include tobacco, amber and musk.
The scents in the top and middle notes are influenced by the base notes; conversely, the scents of the base notes will be altered by the types of fragrance materials used as middle notes. Manufacturers who publish perfume notes typically do so with the fragrance components presented as a fragrance pyramid, using imaginative and abstract terms for the components listed.
Aftershave is a product applied to skin after shaving. Traditionally it is an alcohol based liquid (splash), but it can be a lotion, gel, or even a paste.
It often contains an antiseptic agent such as denatured alcohol, stearate citrate or witch hazel to prevent infection of cuts, as well as to act as an astringent to reduce skin irritation. Menthol is used in some varieties as well to numb irritated skin.
An alcohol-based aftershave usually causes an immediate stinging sensation after applying it post-shave, with effects sometimes lasting several minutes, but most commonly only for seconds. For this reason, a market consisting of highly differentiated products has been created—some using alcohols, some not.
Aftershave balms are frequently recommended for winter use as they tend to be alcohol free and lotion-like, moisturizing the skin.
Some aftershaves use fragrance or essential oil to enhance scent. Moisturizers—natural and artificial, are often touted as able to soften the skin.
Aftershave is sometimes mistakenly referred to as Eau de Cologne due to the very similar nature of the two products. Some aftershave manufacturers encourage using their fragranced aftershave as if it were cologne, in order to increase sales by encouraging consumers to use it in a more versatile manner, rather than just after a shaving session. Some aftershaves were inspired by a cologne.
Early aftershaves included witch-hazel and bay rum, and have been documented in shaving guides. Both still are sold as aftershaves.
Hairy Chemistry
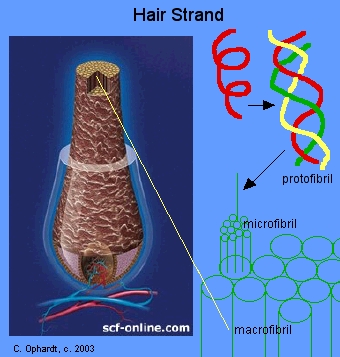
Hair keratin consists of many protein alpha-helices (Figure \(\PageIndex{10}\)). Three alpha-helices are interwoven into a left-handed coil called a protofibril. Eleven protofibrils are bonded and coiled together to make a microfibril. Hundreds of these microfibrils are cemented into an irregular bundle called a macrofibril. These in turn are mixed with dead and living cells to make a complete strand of hair.
The alpha-helices are extensively cross-linked with disulfide bonds from cysteine. These bonds enable keratin to have a somewhat elastic nature. If the alpha -helices stretch unevenly past each other, the disulfide cross-links return them to the original position when the tension is released.
Shampoo
Shampoo (/ʃæmˈpuː/) is a hair care product, typically in the form of a viscous liquid, that is used for cleaning hair. Less commonly, shampoo is available in bar form, like a bar of soap. Shampoo is used by applying it to wet hair, massaging the product into the scalp, and then rinsing it out. Some users may follow a shampooing with the use of hair conditioner.
The typical reason of using shampoo is to remove the unwanted build-up of sebum in the hair without stripping out so much as to make hair unmanageable. Shampoo is generally made by combining a surfactant, most often sodium lauryl sulfate or sodium laureth sulfate, with a co-surfactant, most often cocamidopropyl betaine in water. The sulphate ingredient acts as a surfactant, essentially heavy duty soap that makes it easier to trap oil and grease.
Specialty shampoos are marketed to people with dandruff, color-treated hair, gluten or wheat allergies, an interest in using an organic product, and infants and young children ("baby shampoo" is less irritating). There are also shampoos intended for animals that may contain insecticides or other medications to treat skin conditions or parasite infestations such as fleas.
Shampoo is generally made by combining a surfactant, most often sodium lauryl sulfate or sodium laureth sulfate, with a co-surfactant, most often cocamidopropyl betaine in water to form a thick, viscous liquid. Other essential ingredients include salt (sodium chloride), which is used to adjust the viscosity, a preservative and fragrance. Other ingredients are generally included in shampoo formulations to maximize the following qualities:
- pleasing foam
- ease of rinsing
- minimal skin and eye irritation
- thick or creamy feeling
- pleasant fragrance
- low toxicity
- good biodegradability
- slight acidity (pH less than 7)
- no damage to hair
- repair of damage already done to hair
Many shampoos are pearlescent. This effect is achieved by the addition of tiny flakes of suitable materials, e.g. glycol distearate, chemically derived from stearic acid, which may have either animal or vegetable origins. Glycol distearate is a wax. Many shampoos also include silicone to provide conditioning benefits.
Video \(\PageIndex{2}\) Shampoo applied to wet hair.
Hair Coloring
Hair color is the pigmentation of hair follicles due to two types of melanin: eumelanin and pheomelanin. Generally, if more eumelanin is present, the color of the hair is darker; if less eumelanin is present, the hair is lighter. Levels of melanin can vary over time causing a person's hair color to change, and it is possible to have hair follicles of more than one color on the same person. Particular hair colors are often associated with ethnic groups, while gray or white hair is associated with age.
Hair coloring, or hair dyeing, is the practice of changing the hair color. The main reasons for this are cosmetic: to cover gray or white hair, to change to a color regarded as more fashionable or desirable, or to restore the original hair color after it has been discolored by hairdressing processes or sun bleaching.
Hair coloring can be done professionally by a hairdresser or independently at home. Today, hair coloring is very popular, with 75% of women and 18% of men living in Copenhagen having reported using hair dye (according to a study by the University of Copenhagen). At-home coloring in the United States reached $1.9 billion in 2011 and was expected to rise to $2.2 billion by 2016.
Hair color can be changed by a chemical process. Hair coloring is classed as "permanent" or "semi-permanent".
Permanent hair color means that the hair's structure has been chemically altered until it is eventually cut away. This does not mean that the synthetic color will remain permanently. During the process, the natural color is removed, one or more shades, and synthetic color has been put in its place. All pigments wash out of the cuticle. Natural color stays in much longer and artificial will fade the fastest (depending on the color molecules and the form of the dye pigments).
Permanent hair coloring requires three components: (1) 1,4-diaminobenzene (historically) or 2,5-diaminotoluene (currently), (2) a coupling agent, and (3) an oxidant. The process is typically performed under basic conditions. The mechanism of oxidation dyes involves three steps: 1) Oxidation of 1,4-diaminobenzene derivative to the quinone state. 2) Reaction of this diimine with a coupler compound (more detail below). 3) Oxidation of the resulting compound to give the final dye.

Steps in Permanent Hair Coloring
The first step shows the oxidation of p-phenylenediamine to the quinonediimine (C6H4(NH)2):

The second step involves the attack of this quinonediimine on the coupler.

In the third and final step, the product from the quinonediimine-coupler reaction oxidizes to the final hair dye.

It was once believed that the dye forms in the above reaction bonds to hair permanently. It was later shown that the main reason that this reaction imparts a permanent color on hair by producing larger dye molecules, which is locked inside the hair.
Semi-permanent color washes out over a period of time—typically four to six weeks, so root regrowth is less noticeable. The final color of each strand is affected by its original color and porosity, so there will be subtle variations in color across the head—more natural and less harsh than a permanent dye. However, this means that gray and white hair will not dye to the same color as the rest of the head (in fact, some white hair will not absorb the color at all). A few gray and white hairs will blend in sufficiently not to be noticeable, but as they become more widespread, there will come a point where a semi-permanent alone will not be enough. The move to 100% permanent color can be delayed by using a semi-permanent as a base color, with permanent highlights.
Semi-permanent hair color cannot lighten hair. Hair can only be lightened using chemical lighteners, such as bleach. Bleaching is always permanent because it removes the natural pigment.
"Rinses" are a form of temporary hair color, usually applied to hair during a shampoo and washed out again the next time the hair is wash.
Plant based dyes include henna, indigo and anthocyanin pigments extracted form blackcurrant skin waste.
Video \(\PageIndex{3}\) Applying hair dye.
Permanent and Temporary Waving
Temporary Wave. When the hair gets wet, water molecules intrude into the keratin strands. The sheer numbers of water molecules are able to disrupt some of the hydrogen bonds which also help to keep the alpha-helices aligned. The helices are able to slip past each other and will retain a new shape in the hair drying process as new hydrogen bonds are formed. The hair strands are able for a short time to maintain the new curl in the hair.
A permanent hairstyle, commonly called a perm or "permanent" (sometimes called a "perm" to distinguish it from a "straight perm"), is a hairstyle consisting of styles set into the hair. The hairstyle may last a number of months, hence the name.
Perms may be applied using thermal or chemical means. In the latter method, chemicals are applied to the hair, which is then wrapped around forms to produce hairstyles. The same process is used for chemical straightening or relaxing, with the hair being flattened instead of curled during the chemical reaction.
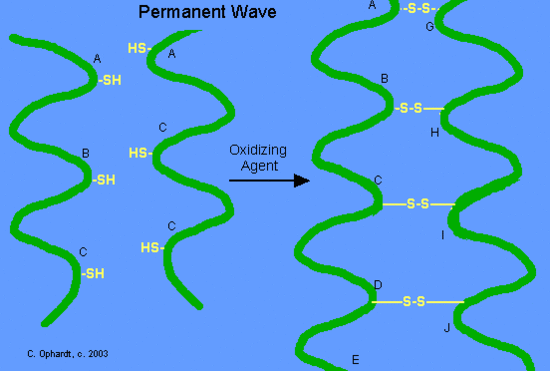
The formation of disulfide bonds has a direct application in producing curls in hair by the permanent wave process. Disulfide bonds Figure \(\PageIndex{12}\) are formed by oxidation of the sulfhydryl groups on cysteine. Different protein chains or loops within a single chain are held together by the strong covalent disulfide bonds. The alpha-helices in the hair strands are bonded by disulfide links.
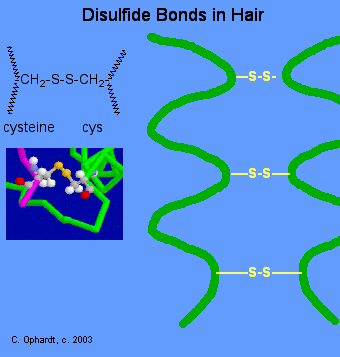
In the permanent wave process, a basic reducing substance (usually ammonium thioglycolate) is first added to reduce and rupture some of the disulfide cross-links, see Figure \(\PageIndex{13}\) below.
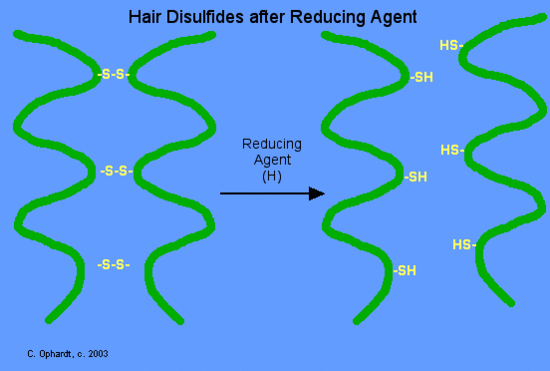
The hair is put on rollers or curlers. Since the alpha-helices are no longer tightly cross-linked to each other, the alpha-helices can shift positions in relation to each other. An oxidizing agent, usually a dilute solution of hydrogen peroxide, (also called the neutralizer) is added to reform the disulfide bonds in their new positions (Figure \(\PageIndex{14}\)). The permanent will hold these new disulfide bond positions until the hair grows out, since new hair growth is of course not treated.
Hair spray
Hair spray is a common household aqueous solution which is used to stiffen hair into a certain style. It was first developed and manufactured in 1948 by Chase Products, based in Broadview, Illinois. Weaker than hair gel or hair wax, it is sprayed directly onto the hair to hold styles for long periods of time. It sprays evenly over the hair using a pump or aerosol spray nozzle. The product may leave hair feeling 'crunchy' unless brushed out.
The active ingredients in hair spray are called polymers, which keep the hair stiff and firm without snapping. Solvents, which make up most of the content of the hairspray, are responsible for carrying these polymers in a solution.
Originally, the solvent found in hair spray was a chlorofluorocarbon (CFC). CFCs are nontoxic, nonflammable, and make almost ideal aerosol propellants. However, when research concluded that CFCs cause destruction of stratospheric ozone, they were replaced with other solvents, such as alcohols and hydrocarbons.
Hair sprays consist of the following components: concentrate, plasticizers, luster agents, and fragrances, as well as propellants.
One of the polymers used in hair spray is polyvinylpyrrolidone (Figure \(\PageIndex{15}\)) , which is water-soluble. The non-water-soluble polymer polydimethylsiloxane is added to make the hold last a bit longer. Some less common polymers found in hair spray include copolymers with vinyl acetate and copolymers with maleic anhydride.

Some hair sprays use natural polymers and solvents like vegetable gums dissolved in alcohol. One popular ingredient in natural hair sprays is gum arabic, which is made from the sap of various species of the acacia tree. Gum tragacanth is another herbal gum that is used to stiffen calico and crepe, as well as hair.
Hair Removers
Depilation is the removal of the part of the hair above the surface of the skin. The most common form of depilation is shaving or trimming. Another option is the use of chemical depilatories, which work by breaking the disulfide bonds that link the protein chains that give hair its strength.
A chemical depilatory is a cosmetic preparation used to remove hair from the skin. Common active ingredients are salts of thioglycolic acid and thiolactic acids. These compounds break the disulfide bonds in keratin and also hydrolyze the hair so that it is easily removed. Formerly, sulfides such as strontium sulfide were used, but due to their unpleasant odor, they have been replaced by thiols.
The main chemical reaction effected by the thioglycolate is:
2 HSCH2CO2H (thioglycolic acid) + R-S-S-R (cystine) → HO2CCH2-S-S-CH2CO2H (dithiodiglycolic acid) + 2 RSH (cysteine)
Chemical depilatories contain 5–6% calcium thioglycolate in a cream base (to avoid runoff). Calcium hydroxide or strontium hydroxide maintain a pH of about 12. Hair destruction requires about 10 minutes. Depilation is followed by careful rinsing with water, and various conditioners are applied to restore the skin's pH to normal. Depilation does not destroy the dermal papilla, and the hair grows back.
Chemical depilatories are available in gel, cream, lotion, aerosol, roll-on, and powder forms. Common brands include Nair, Magic Shave and Veet.
Depilatory ointments, or plasters, were known to Greek and Roman authors as psilothrum. In Jewish lore, King Solomon is said to have discovered a chemical depilatory made from a mixture of lime and water and orpiment (arsenic trisulfide).
Hair Restorers
Treatments for the various forms of hair loss have only moderate success. Three medications have evidence to support their use in male pattern hair loss: finasteride, dutasteride and minoxidil. They typically work better to prevent further hair loss than to regrow lost hair.
They may be used together when hair loss is progressive or further regrowth is desired after 12 months. Other medications include ketoconazole, and in female androgenic alopecia spironolactone and flutamide. Combinations of finasteride, minoxidil and ketoconazole are more effective than individual use.
Minoxidil is applied topically, is widely used for the treatment of hair loss. It may be effective in helping promote hair growth in both men and women with androgenic alopecia. About 40% of men experience hair regrowth after 3–6 months. It is the only topical product that is FDA approved in America for androgenic hair loss. However, increased hair loss has been reported.
Finasteride is used to treat male pattern hair loss. Treatment provides about 30% improvement in hair loss after six months of treatment, and effectiveness only persists as long as the drug is taken. There is no good evidence for its use in women. It may cause gynecomastia, erectile dysfunction and depression.
Dutasteride is used off label for male pattern hair loss.
There is tentative support for spironolactopne in women. Due to its feminising side effects and risk of infertility it is not often used by men. It can also cause low blood pressure, high blood potassium, and abnormal heart rhythms. Also, women who are pregnant or trying to become pregnant generally cannot use the medication as it is a teratogen, and can cause ambiguous genitalia in newborn children.
There is tentative evidence for flutamide in women; however, it is associated with relatively high rates of liver problems. Like spironolactone, it is typically only used by women.
Ketoconazole shampoo in conjunction with an oral 5α-reductase inhibitor such as finasteride or dutasteride has been used off label to treat androgenic alopecia.
Summary
- Various personal care products contain ingredients to protect the skin, protect the hair, promote hygiene, for aesthetic purposes etc.
- Temporary and permanent waves are formed due to the disruption and reformation of disulfide bonds in hair strands.
Contributors
- US FDA
- American Academy of Dermatology Association
- Libretext: Anatomy and Physiology 1 (Lumen)
- Libretext: Human Biology (Wakim and Grewal)
- Libretext: Supplemental Module (Biological Chemistry)
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook

