9.8: Nitrogen-Containing Compounds- Amines and Amides
- Page ID
- 153840
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the structure and properties of amines and amides.
- Name simple amines and amides.
The addition of nitrogen into an organic framework leads to two families of molecules. Compounds containing a nitrogen atom bonded in a hydrocarbon framework are classified as amines. Compounds that have a nitrogen atom bonded to one side of a carbonyl group are classified as amides.
Amines
An amine is an organic compound that can be considered to be a derivative of ammonia \(\left( \ce{NH_3} \right)\). Amines are molecules that contain carbon-nitrogen bonds. Amines are named by naming the alkyl groups attached to the nitrogen atom, followed by the suffix -amine as illustrated here for a few simple examples:
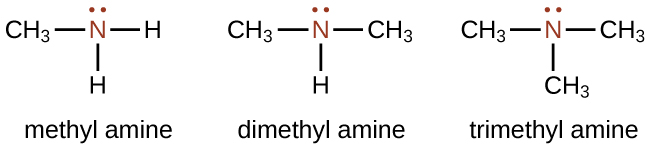
The name of larger molecules involve the class-identifying suffix –ine as you will see later in this section(e.g. caffeine and nicotine).
Methylamine and ethylamine are gases at room temperature, while larger amines are liquids. As with other organic compounds that form hydrogen bonds, water solubility is reflected in the length of the carbon chains. Smaller amines are soluble, while larger ones are less soluble.
Amines generally have rather pungent or noxious odors. Ammonia can be considered the simplest amine and has a very distinctive odor. Methylamine has an unpleasant odor associated with dead fish. Amines are often formed biologically during the breakdown of proteins in animal cells, and so many have the smell of death and decay. Putrescine and cadaverine are two such amines and are aptly named for their foul odors. The toxins which many animals use as a form of defense are frequently amines. Amines are used industrially as dyes and in many drugs.
Amines have “interesting” odors. The simple ones smell very much like ammonia. Higher aliphatic amines smell like decaying fish. Or perhaps we should put it the other way around: Decaying fish give off odorous amines. The stench of rotting fish is due in part to two diamines: putrescine and cadaverine. They arise from the decarboxylation of ornithine and lysine, respectively, amino acids that are found in animal cells.
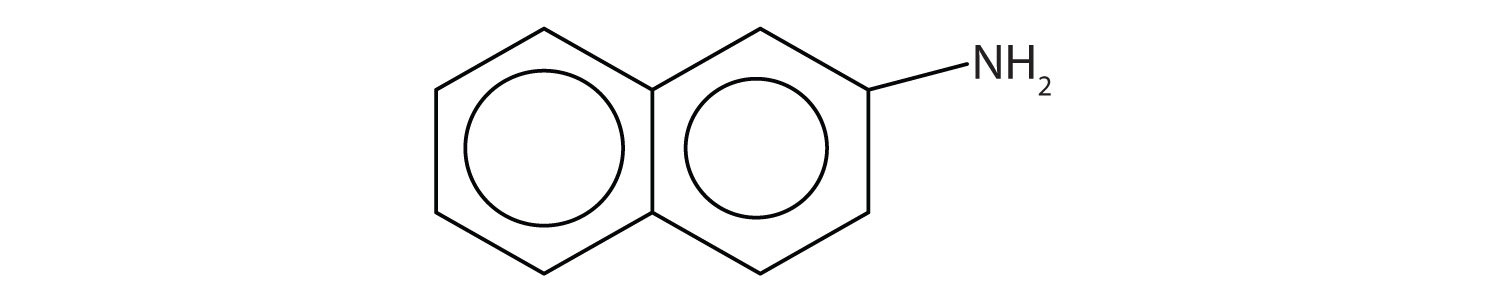
Aromatic amines generally are quite toxic. They are readily absorbed through the skin, and workers must exercise caution when handling these compounds. Several aromatic amines, including β-naphthylamine, are potent carcinogens.
Amides
The amide functional group has an nitrogen atom attached to a carbonyl carbon atom. If the two remaining bonds on the nitrogen atom are attached to hydrogen atoms, the compound is a simple amide. If one or both of the two remaining bonds on the atom are attached to alkyl or aryl groups, the compound is a substituted amide.
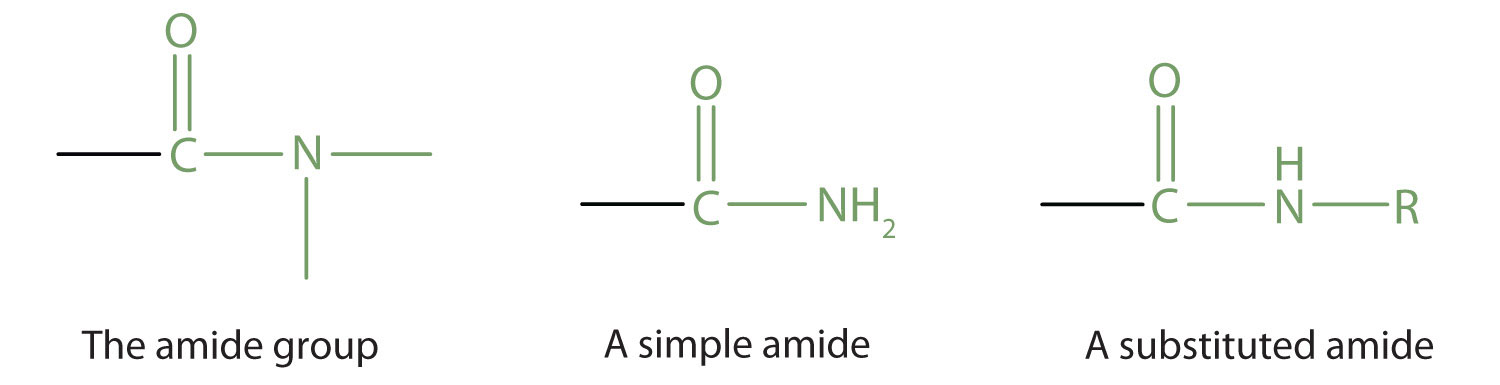
Simple amides are named as derivatives of carboxylic acids. The -ic ending of the common name or the -oic ending of the International Union of Pure and Applied Chemistry (IUPAC) name of the carboxylic acid is replaced with the suffix -amide.

Amides can be produced when carboxylic acids react with amines or ammonia in a process called amidation. A water molecule is eliminated from the reaction, and the amide is formed from the remaining pieces of the carboxylic acid and the amine (note the similarity to formation of an ester from a carboxylic acid and an alcohol discussed in the previous section):
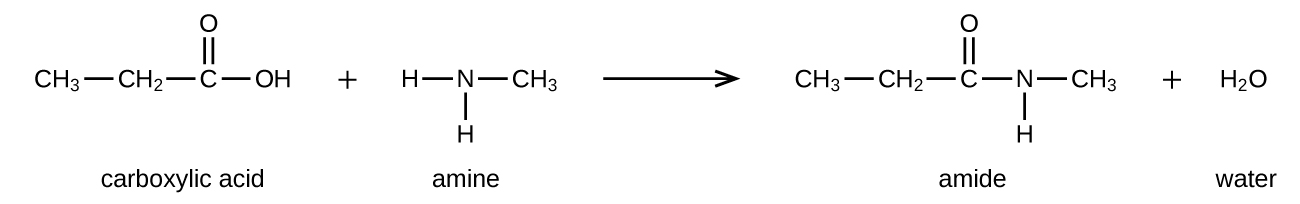
The reaction between amines and carboxylic acids to form amides is biologically important. It is through this reaction that amino acids (molecules containing both amine and carboxylic acid substituents) link together in a polymer to form proteins.
The carbonyl carbon-to-nitrogen bond is called an amide linkage. This bond is quite stable and is found in the repeating units of protein molecules, where it is called a peptide linkage.
Amides are pervasive in nature and technology as structural materials. The amide linkage is easily formed, confers structural rigidity, and resists hydrolysis. Nylons are polyamides, as are the very resilient materials Aramid, Twaron, and Kevlar. Amide linkages constitute a defining molecular feature of proteins, the secondary structure of which is due in part to the hydrogen bonding abilities of amides. Amide linkages in a biochemicalcontext are called peptide bonds when they occur in the main chain of a protein and isopeptide bonds when they occur to a side-chain of the protein. Proteins can have structural roles, such as in hair or spider silk, but also nearly all enzymes are proteins. Low molecular weight amides, such as dimethylformamide (HC(O)N(CH3)2), are common solvents. Many drugs are amides, including paracetamol, penicillin and LSD. Moreover, plant N-alkylamides have a wide range of biological functionalities.
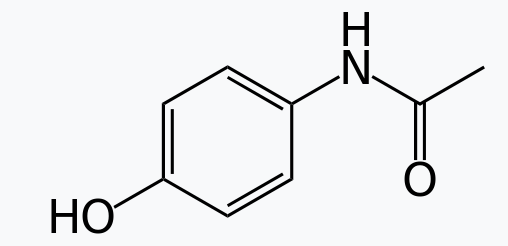
(Figure \(\PageIndex{3}\)) Paracetamol (acetaminophen) LSD (Lysergic diethylamide)
Heterocyclic Compounds: Alkaloids and Others
Looking back at the various cyclic hydrocarbons discussed previously, we see that all the atoms in the rings of these compounds are carbon atoms. In other cyclic compounds, called heterocyclic compounds (Greek heteros, meaning “other”), nitrogen, oxygen, sulfur, or some other atom is incorporated in the ring. Many heterocyclic compounds are important in medicine and biochemistry. Some compose part of the structure of the nucleic acids, which in turn compose the genetic material of cells and direct protein synthesis.
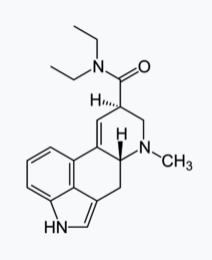
Many heterocyclic amines occur naturally in plants. Like other amines, these compounds are basic. Such a compound is an alkaloid, a name that means “like alkalis.” Many alkaloids are physiologically active, including the familiar drugs caffeine, nicotine, and cocaine.
Caffeine is a stimulant found in coffee, tea, and some soft drinks. Its mechanism of action is not well understood, but it is thought to block the activity of adenosine, 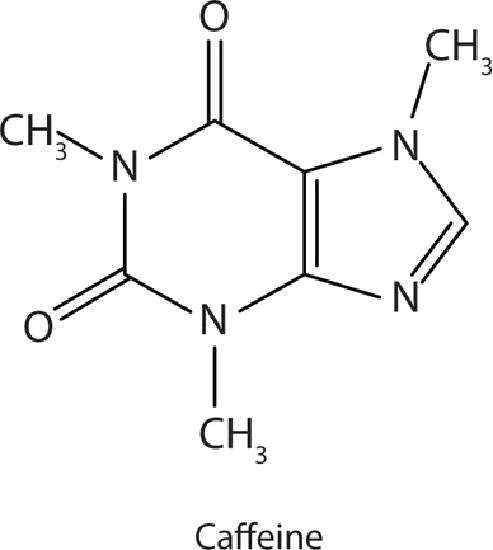 a heterocyclic base that acts as a neurotransmitter, a substance that carries messages across a tiny gap (synapse) from one nerve cell (neuron) to another cell. The effective dose of caffeine is about 200 mg, corresponding to about two cups of strong coffee or tea.
a heterocyclic base that acts as a neurotransmitter, a substance that carries messages across a tiny gap (synapse) from one nerve cell (neuron) to another cell. The effective dose of caffeine is about 200 mg, corresponding to about two cups of strong coffee or tea.
Nicotine acts as a stimulant by a different mechanism; it probably mimics the action of the 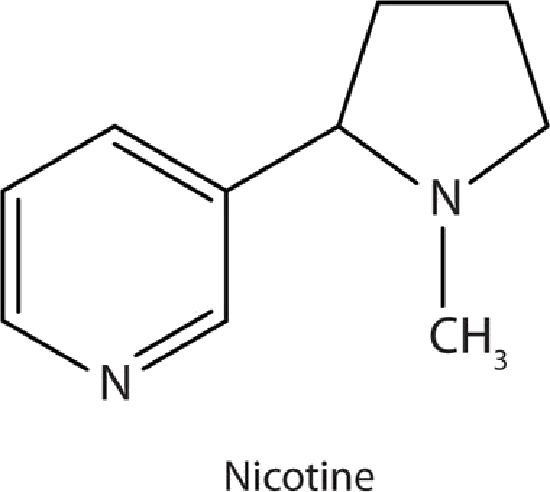 neurotransmitter acetylcholine. People ingest this drug by smoking or chewing tobacco. Its stimulant effect seems transient, as this initial response is followed by depression. Nicotine is highly toxic to animals. It is especially deadly when injected; the lethal dose for a human is estimated to be about 50 mg. Nicotine has also been used in agriculture as a contact insecticide.
neurotransmitter acetylcholine. People ingest this drug by smoking or chewing tobacco. Its stimulant effect seems transient, as this initial response is followed by depression. Nicotine is highly toxic to animals. It is especially deadly when injected; the lethal dose for a human is estimated to be about 50 mg. Nicotine has also been used in agriculture as a contact insecticide.
Cocaine acts as a stimulant by preventing nerve cells from taking up dopamine, another neurotransmitter, from the synapse. High levels of dopamine are therefore 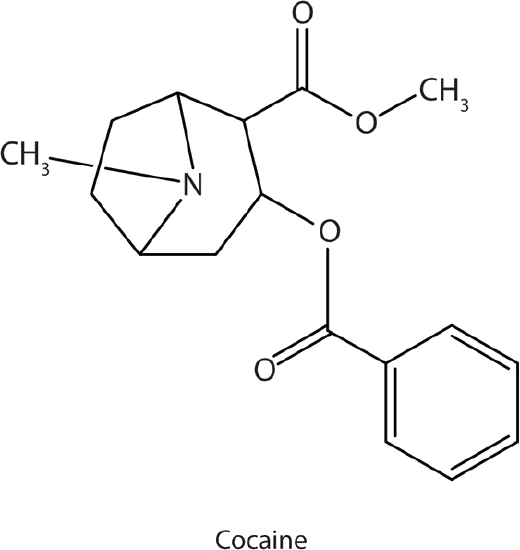 available to stimulate the pleasure centers of the brain. The enhancement of dopamine action is thought to be responsible for cocaine’s “high” and its addictive properties. After the binge, dopamine is depleted in less than an hour. This leaves the user in a pleasureless state and (often) craving more cocaine.
available to stimulate the pleasure centers of the brain. The enhancement of dopamine action is thought to be responsible for cocaine’s “high” and its addictive properties. After the binge, dopamine is depleted in less than an hour. This leaves the user in a pleasureless state and (often) craving more cocaine.
Cocaine is used as the salt cocaine hydrochloride and in the form of broken lumps of the free (unneutralized) base, which is called crack cocaine.
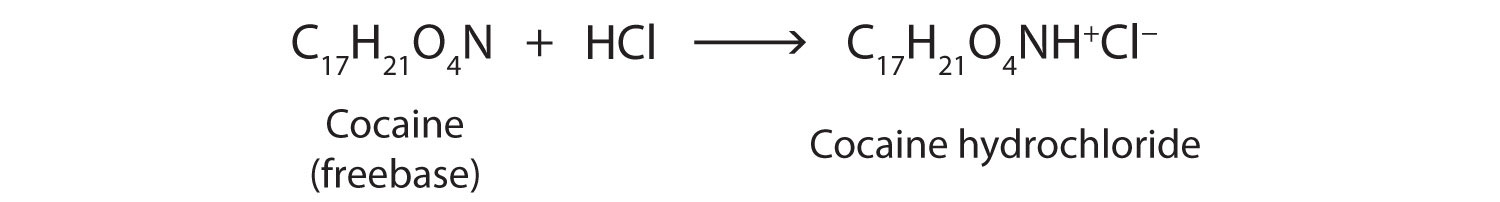
Because it is soluble in water, cocaine hydrochloride is readily absorbed through the watery mucous membranes of the nose when it is snorted. Crack cocaine is more volatile than cocaine hydrochloride. It vaporizes at the temperature of a burning cigarette. When smoked, cocaine reaches the brain in 15 s.
Morphine, a strong narcotic used to relieve pain, contains two hydroxyl functional 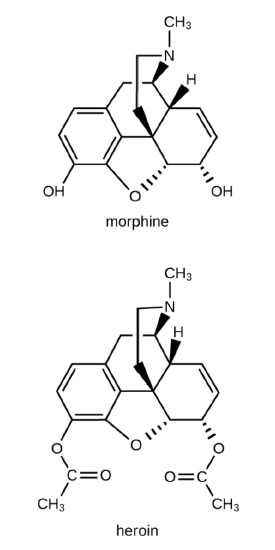 groups, located at the bottom of the
groups, located at the bottom of the

Identify whether each compound is an amide or an amine.
a. 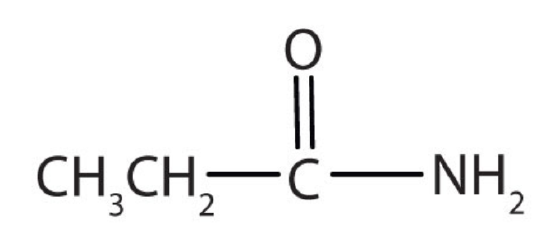
b. 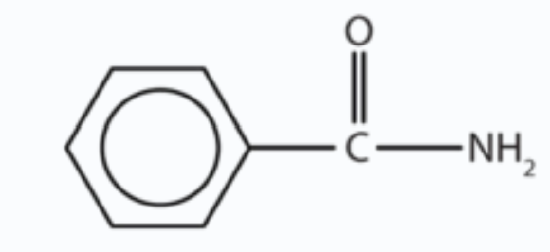
c. 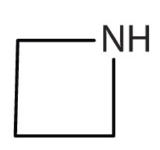
Solutions
a. The compound has the CONH2 functional group so it is an amide.
b. The compound has the CONH2 functional group so it is an amide.
c. The compound has the NH functional group so it is an amine.
a. 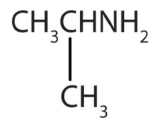
b. CH3CH2CH2CH2CH2CH2NH2
c. 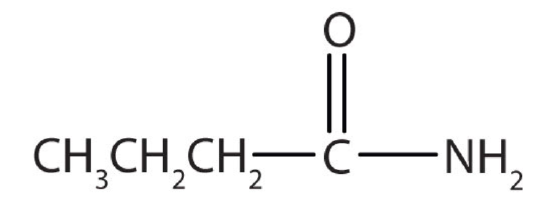
- Answer
-
a. amine b. amine c. amide
Summary
- An amine is a derivative of ammonia in which one, two, or all three hydrogen atoms are replaced by hydrocarbon groups. The amine functional group is as follows:
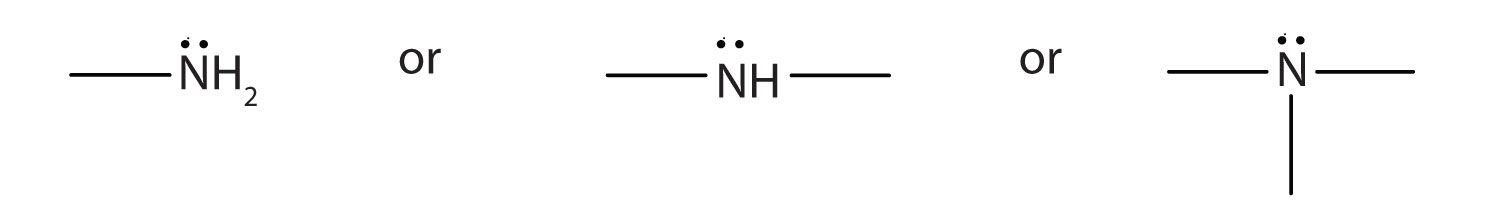
- Amines are named by naming the alkyl groups attached to the nitrogen atom, followed by the suffix -amine.
- Amides have a general structure in which a nitrogen atom is bonded to a carbonyl carbon atom.
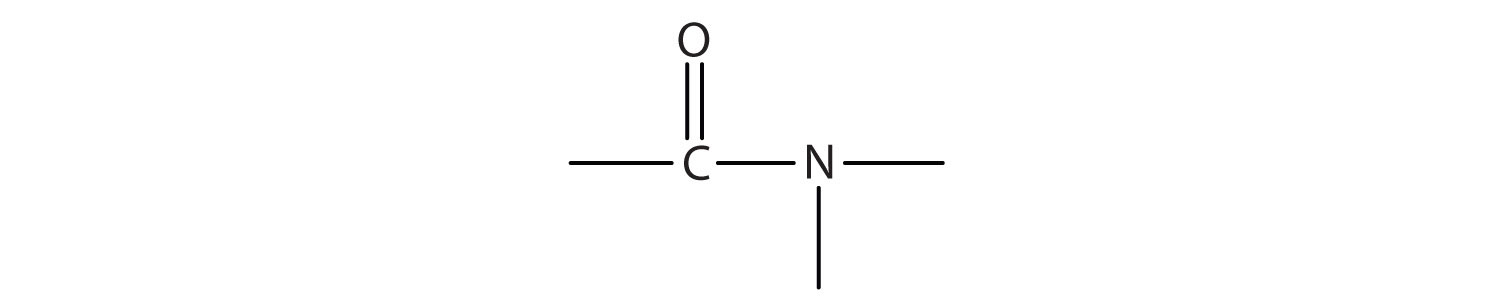
- Like amines, various nomenclature rules may be used to name amides, but all include use of the class-specific suffix -amide:
- Heterocyclic amines are cyclic compounds with one or more nitrogen atoms in the ring.
Contributors and Attributions
Marisa Alviar-Agnew (Sacramento City College)
Libretext: The Basics of GOB Chemistry (Ball et al.)
OpenSTAX
Wikipedia

