6.19: Periodic Trends - Electron Affinity
- Page ID
- 53710
Do you tend to overpack before going on trips?
Packing for a trip can be very challenging. What do you take with you? Where will you be going and what will you need? We usually pack too much (like the suitcase above) and then find it hard to close the suitcase. When the suitcase is over-full, there is stress on the system and forces pushing the suitcase open. When electrons are added to an atom, the increased negative charge puts stress on the electrons already there, causing energy to be released.
When electrons are removed from an atom, that process requires energy to pull the electron away from the nucleus. Addition of an electron releases energy from the process.
Electron Affinity
In most cases, the formation of an anion by the addition of an electron to a neutral atom releases energy. This can be shown for the chloride ion formation below:
\[\ce{Cl} + \ce{e^-} \rightarrow \ce{Cl^-} + \: \text{energy}\nonumber \]
The energy change that occurs when a neutral atom gains an electron is called its electron affinity. When energy is released in a chemical reaction or process, that energy is expressed as a negative number. The figure below shows electron affinities in \(\ce{kJ/mol}\) for the representative elements. Electron affinities are measured on atoms in the gaseous state and are very difficult to measure accurately.
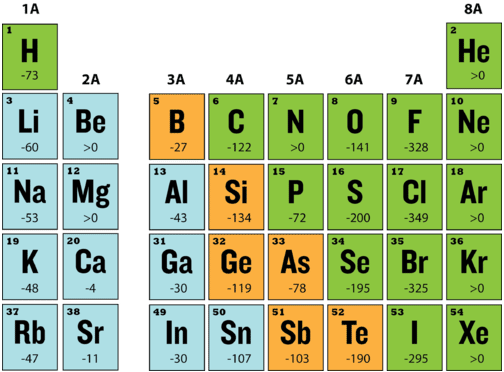
The elements of the halogen group (Group 17) gain electrons most readily, as can be seen from their large negative electron affinities. This means that more energy is released in the formation of a halide ion than for the anions of any other elements. Considering electron configuration, it is easy to see why. The outer configuration of all halogens is \(ns^2 \: np^5\). The addition of one more electron gives the halide ions the same electron configuration as a noble gas, which we have seen is particularly stable.
Period and group trends for electron affinities are not nearly as regular as for ionization energy. In general, electron affinities increase (become more negative) from left to right across a period and decrease (become less negative) from top to bottom down a group. However, there are many exceptions, owing in part to inherent difficulties in accurately measuring electron affinities.
Summary
- Electron affinity is a measure of the energy released when an extra electron is added to an atom.
- Electron affinities are measured in the gaseous state.
- In general, electron affinities become more negative as we move from left to right on the periodic table.
- In general, electron affinities become less negative from top to bottom of a group.
Review
- Define “electron affinity."
- Does addition of an electron to a neutral atom require energy to be absorbed or released?
- Describe the general trend for electron affinity values moving from left to right on the periodic table.
- Describe the general trend for electron affinity values moving from top to bottom in a group on the periodic table.
- Why is more energy released in the formation of a halide ion than with other elements?

