6.18: Electron Shielding
- Page ID
- 53709
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)What is the goal of a roller derby game?
Roller derby is a popular sport, although it is unfamiliar to many people. The basic purpose is to set one team member (the "jammer") past the opposing team to score points. Other members of the team serve as blockers to prevent the opposing team from stopping the jammer. Blockers interfere with the interaction between the jammer and the opponents by getting between the jammer and the skaters trying to stop them.
The attraction between an electron and the nucleus of the atom is not a simple issue. Only with hydrogen is there a one-to-one relationship that can be discussed in terms of direct charge attraction. As the size of the atom increases, the number of protons and electrons also increase. These changes influence how the nucleus attracts electrons.
Electron Shielding
In general, the ionization energy of an atom will increase as we move from left to right across the periodic table. There are several exceptions to the general increase in ionization energy across a period. The elements of Group 13 (\(\ce{B}\), \(\ce{Al}\), etc.) have lower ionization energies than the elements of Group 2 (\(\ce{Be}\), \(\ce{Mg}\), etc.). This is an illustration of a concept called "electron shielding". Outer electrons are partially shielded from the attractive force of the protons in the nucleus by inner electrons.
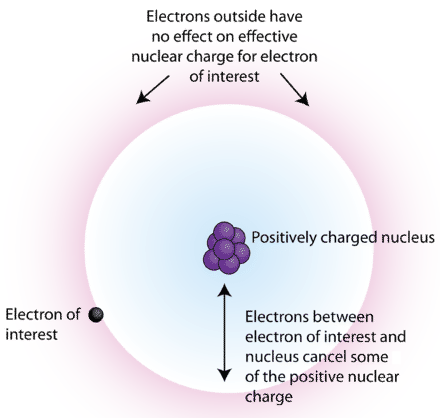
To explain how shielding works, consider a lithium atom. It has three protons and three electrons—two in the first principal energy level and its valence electron in the second. The valence electron is partially shielded from the attractive force of the nucleus by the two inner electrons. Removing that valence electron becomes easier because of the shielding effect.
There is also a shielding effect that occurs between sublevels within the same principal energy level. Specifically, an electron in the \(s\) sublevel is capable of shielding electrons in the \(p\) sublevel of the same principal energy level. This is because of the spherical shape of the \(s\) orbital. The reverse is not true—electrons in the \(p\) orbitals do not shield electrons in \(s\) orbitals.
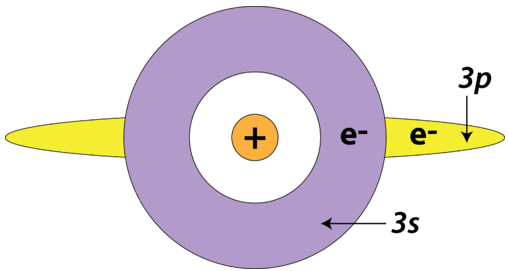
The electron being removed from an \(\ce{Al}\) atom is a \(3p\) electron, which is shielded by the two \(3s\) electrons, as well as all the inner core electrons. The electron being removed from a \(\ce{Mg}\) atom is a \(3s\) electron, which is only shielded by the inner core electrons. Since there is a greater degree of electron shielding in the \(\ce{Al}\) atom, it is slightly easier to remove the valence electron; its ionization energy is less than that of \(\ce{Mg}\). This is despite the fact that the nucleus of the \(\ce{Al}\) atom contains one more proton than the nucleus of the \(\ce{Mg}\) atom.
There is another anomaly between Groups 15 and 16. Atoms of Group 16 (\(\ce{O}\), \(\ce{S}\), etc.) have lower ionization energies than atoms of Group 15 (\(\ce{N}\), \(\ce{P}\), etc.). Hund's rule is behind the explanation. In a nitrogen atom, there are three electrons in the \(2p\) sublevel and each is unpaired. In an oxygen atom, there are four electrons in the \(2p\) sublevel, so one orbital contains a pair of electrons. It is that second electron in the orbital that is removed in the ionization of an oxygen atom. Since electrons repel each other, it is slightly easier to remove the electron from the paired set in the oxygen atom than it is to remove an unpaired electron from the nitrogen atom.
Summary
- Electron shielding refers to the blocking of valence shell electron attraction by the nucleus, due to the presence of inner-shell electrons.
- Electrons in an \(s\) orbital can shield \(p\) electrons at the same energy level because of the spherical shape of the \(s\) orbital.
- Electrons in paired spin configurations are slightly easier to remove than unpaired electrons.
Review
- Define “electron shielding."
- Why do group 13 elements have lower ionization energies than group 2 elements?
- What influence does a larger shielding effect have on ionization energy?
- How do s orbit electrons affect the ionization energy of a p electron in the same shell?
- Why do group 16 atoms have lower ionization energies than the corresponding group 15 atoms?

