Hard Water
- Page ID
- 601
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Hard water contains high amounts of minerals in the form of ions, especially the metals calcium and magnesium, which can precipitate out and cause problems in water cconducting or storing vessels like pipes. Hard water can be distinguished from other types of water by its metallic, dry taste and the dry feeling it leaves on skin. It is responsible for the scum rings seen in bathtubs, as well as the inability of soap to lather.
Types of Hard Water

Hard water is water containing high amounts of mineral ions. The most common ions found in hard water are the metal cations calcium (Ca2+) and magnesium (Mg2+), though iron, aluminum, and manganese may also be found in certain areas. These metals are water soluble, meaning they will dissolve in water. The relatively high concentrations of these ions can saturate the solution and consequently cause the equilibrium of these solutes to shift to the left, towards reactants. In other words, the ions can precipitate out of the solution. This displacement of minerals from the solution is responsible for the calcination often seen on water faucets, which is a precipitation of calcium or magnesium carbonate. Hard water may also react with other substances in the solution, such as soap, and form a precipitate called "scum." There are two defined types of hard water, temporary and permanent, which are described below.
Temporary Hard Water
Temporary hard water is hard water that consists primarily of calcium (Ca2+) and bicarbonate (HCO3-) ions. Heating causes the bicarbonate ion in temporary hard water to decompose into carbonate ion (CO32-), carbon dioxide (CO2), and water (H2O). The resultant carbonate ion (CO32-) can then react with other ions in the solution to form insoluble compounds, such as CaCO3 and MgCO3. The interactions of carbonate ion in the solution also cause the well-known mineral build-up seen on the sides of pots used to boil water, a rust known as "boiler scale." Increasing the temperature of temporary hard water, with its resultant decomposition of the bicarbonate ion, signifies a shift in the equilibrium equation (shown below). The high temperature causes the equilibrium to shift to the left, causing precipitation of the initial reactants.
\[CaCO_{3 \; (s)} + CO_{2 \; (aq)} + H_2O_{(l)} \rightleftharpoons Ca^{2+}_{(aq)} + 2HCO^-_{3 \; (aq)} \tag{1} \]
This shift is responsible for the white scale observed in the boiling containers described above, as well as the mineral deposits that build up inside water pipes, resulting in inefficiency and even explosion due to overheating. The CaCO3 or other scale does not completely dissolve back into the water when it is cooled because it is relatively insoluble, as shown by its small solubility constant. For this reason, this type of hard water is "temporary" because boiling can remove the hardness by displacing the offending ions from solution.
\[CaCO_{3 \; (s)} \rightleftharpoons Ca^{2+}_{(aq)} + CO^{2-}_{3 \; (aq)} \tag{2a} \]
\[K_{(sp)} = 2.8 \times 10^{-9} \tag{2b} \]
Permanent Hard Water
Permanent hard water consists of high concentrations of anions, like the sulfate anion (SO42-). This type of hard water is referred to as "permanent" because, unlike temporary hard water, the hardness cannot be removed simply by boiling the water and thereby precipitating out the mineral ions. However, the name is deceiving as "permanent" hard water can be softened by other means. The scale caused by permanent hard water has detrimental effects similar to those seen with temporary hard water, such as obstruction of water flow in pipes. Permanent hard water is also responsible for the bathtub "ring," or soap scum, seen after showering or bathing. As previously mentioned, permanent hard water contains calcium and magnesium cations.These cations react with soap to form insoluble compounds that are then deposited on the sides of the tub. Additionally, the reaction of these cations with soap is the reason it is difficult for soap to foam or lather well in hard water. The equation below gives an example of the reaction of magnesium ion with components of soap, in this case stearate (C18H35O22-), to form the insoluble compound magnesium stearate, which is responsible for the infamous soap scum.
\[2(C_{18}H_{35}O_2)^{2-}_{(aq)} + Mg^{2+}_{(aq)} \longrightarrow Mg(C_{18}H_{35}O_2)_{2 \; (s)} \tag{3} \]
Effects on the body
Though the taste of hard water may be unpleasant to some, it has many health benefits when compared to soft water. Two of the most prevalent minerals in hard water are calcium and magnesium. Both calcium and magnesium are considered essential nutrients, meaning that they must be provided in the diet in order to maintain healthy body function. Calcium is a critical component of bones, and has many positive effects on the body, such as prevention of serious life-threatening and painful ailments like osteoporosis, kidney stones, hypertension, stroke, obesity, and coronary artery disease. Magnesium also has positive health effects. Inadequate amounts of magnesium in the body increase the risks for some forms of health problems, such as hypertension, cardiac arrhythmia, coronary heart disease, and diabetes mellitus. Studies done on the health effects of hard and soft water have shown that people who drink greater amounts of soft water have much higher incidences of heart disease, as well as higher blood pressure and cholesterol levels, and faster heart rates than those who drink mostly hard water. Furthermore, soft water is corrosive to pipes, which may allow for toxic substances like lead to contaminate drinking water.
How to soften hard water
Some wish to soften hard water to control its irritating, and in many cases damaging, effects. The diminished ability of soap to lather is not only annoying, but can also be potentially harmful economically. Businesses that depend on the foaming of soap, such as car washes and pet groomers, may wish to soften hard water to avoid excessive use of soap due to a decreased ability to lather. Likewise, it is often necessary to soften water that comes into contact with pipes to avoid the destructive and compromising build-up of deposits. Also, many people may find the calcifying effects that hard water has on faucets and other items unfavorable and choose to soften the water to prevent such mineral deposits from forming. Still others may dislike the sticky, dry feeling left by the precipitation of soap scum onto the skin. Whatever the reasons, there are many processes available to soften hard water.
Ion Exchange
One way to soften water is through a process called ion exchange. During ion exchange, the unwanted ions are "exchanged" for more acceptable ions. In many cases, it is desirable to replace the hard water ions, such as Ca2+ and Mg2+, with more agreeable ions, like that of Na+. To do this, the hard water is conducted through a zeolite or resin-containing column, which binds the unwanted ions to its surface and releases the more tolerable ions. In this process, the hard water ions become "fixed" ions because of their attachment to the resin material. These fixed ions displace the desirable ions (Na+), now referred to as counterions, from the column, thus exchanging the ions in the water. This process is illustrated in Figure 1.
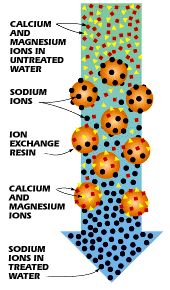
Unfortunately, this process has the disadvantage of increasing the sodium content of drinking water, which could be potentially hazardous to the health of people with sodium-restricted diets.
Lime Softening
Another process is called lime softening. In this process, the compound calcium hydroxide, Ca(OH)2, is added to the hard water. The calcium hydroxide, or "slaked lime," raises the pH of the water and causes the calcium and magnesium to precipitate into CaCO3 and Mg(OH)2. These precipitates can then be easily filtered out due to their insolubility in water, shown below by the small solubility constant of magnesium hydroxide (the solubility product constant for calcium carbonate is shown above). After precipitation and removal of the offending ions, acid is added to bring the pH of the water back to normal.
\[Mg(OH)_{2 \; (s)} \rightleftharpoons Mg^{2+}_{(aq)} + 2OH^-_{(aq)} \tag{4a} \]
\[K_{(sp)} = 1.8 \times 10^{-11} \tag{4b} \]
Chelation
Chelating agents can also be used to soften hard water. Polydentate ligands, such as the popular hexadentate ligand EDTA, bind the undesirable ions in hard water. These ligands are especially helpful in binding the magnesium and calcium cations, which as already mentioned are highly prevalent in hard water solutions. The chelating agent forms a very stable ring complex with the metal cations, which prevents them from interacting with any other substances that may be introduced to the solution, such as soap. In this way, chelators are able to diminish the negative effects associated with hard water. A simplified equation representing the chelation of the metal calcium cation (Ca2+) with the hexadentate ligand EDTA is shown below. The large value of the formation constant (Kf) reflects the tendency of the reaction to proceed to completion in the forward direction.
\[Ca^{2+} + EDTA^{4-} \longrightarrow [Ca(EDTA)]^{2-} \tag{5a} \]
\[K_f = 4.9 \times 10^{10} \tag{5b} \]
Reverse Osmosis
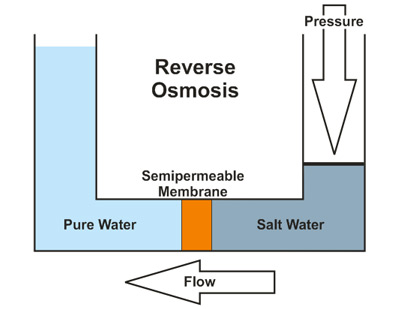
The final process, reverse osmosis, uses high pressures to force the water through a semipermeable membrane. This membrane is generally intended to be impermeable to anything other than water. The membrane serves to filter out the larger ions and molecules responsible for the water's hardness, resulting in softened water. During this process, the water is forced from an area with a high concentration of solute in the form of dissolved metal ions and similar compounds, to an area that is very low in the concentration of these substances. In other words, the water moves from a state of hardness to a softer composition as the ions causing the water's hardness are prevented passage through the membrane. Reverse Osmosis does have a disadvantage of wasting wastewater compared to other water treatment methods. This process is shown in Figure 2 below. Note that this figure describes the desalination of salt water. However, the process for softening hard water is the same.
Practice Problems
1. Name the two main types of hard water. (Highlight blue area for the answers)
Temporary and Permanent.
2. What makes "hard" water hard?
The presence of high concentrations of minerals, typically in the form of metal cations.
3. What are the two most prevalent ions in hard water? How are these important to the proper function of the body?
Calcium (Ca2+) and magnesium (Mg2+) ion. They are important because both are essential nutrients, which means they are necessary for the proper function of the body and are also important for the prevention of many diseases and other ailments.
4. Name the four described processes for softening hard water. It is also important to understand the essential steps of each process.
Ion exchange, chelation, lime softening, and reverse osmosis
5. What is a major disadvantage of ion exchange when Na+ is used as a counterion?
It increases the concentration of sodium in the water, a potential hazard for people with sodium-restricted diets.
References
- Calcium and Magnesium in Drinking Water: public health significance. Geneva, Switzerland: WHO press. 2009.
- Health Effects of Drinking Water Treatment Technologies. Chelsea, MI: Lewis Publishers. 1989.
- Lewis Alan, Scott. Safe Drinking Water. San Francisco, CA: Scott Alan Lewis. 1996.
- Crawford, T. and M. Crawford (1967). "Prevalence and pathological changes of ischaemic heart-disease in a hard-water and in a soft-water area." The Lancet 289(7484): 229-232.
- Stitt, F., D. Clayton, et al. (1973). "Clinical and biochemical indicators of cardiovascular disease among men living in hard and soft water areas." The Lancet 301(7795): 122-126.
- Gardiner, J. (1976). "Complexation of trace metals by ethylenediaminetetraacetic acid (EDTA) in natural waters." Water Research 10(6): 507-514.
Contributors
- Andrea Kubisch, Courtney Korff (UCD)

