8.3: Group 1, The Alkali Metals
- Page ID
- 151403
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The Alkali Metals
The alkali metals comprise the elements lithium through francium in group 1 of the periodic table, as shown in Figure \( {\PageIndex{1}}\).

Alkali metals are powerful reductants and so do not exist as the free metal in the relatively oxidizing environment at the earth's surface. As a result they are commonly found as the +1 cations in a variety of minerals like rock salt (halite, NaCl) and natron (Na2CO3·10H2O).
Because they occur as minerals, free alkali metals are prepared by electrolytic reduction of their +1 cations. For example, in the Downs process for making sodium, NaCl is electrolyzed to Na(l) and Cl2(g).
\[ \ce{2 NaCl(l) \rightarrow 2 Na(l) + Cl_2(g)} \nonumber \]
In order for this process to occur rapidly, transport of cations to the cathode and anions to the anode must occur. To permit this, the salt to be electrolyzed is melted.1 In addition, since the alkali metal is reactive the electrolysis must take place in a specialized cell that permits separation of the metal from both the salt and any oxidation products formed at the anode. For example, to prevent the explosively exothermic reaction between the chlorine gas and molten sodium products in the Downs process, specialized Downs cells in which the cathode and anode are carefully separated are employed. A typical way Downs cell is depicted in Figure \( {\PageIndex{2}}\).
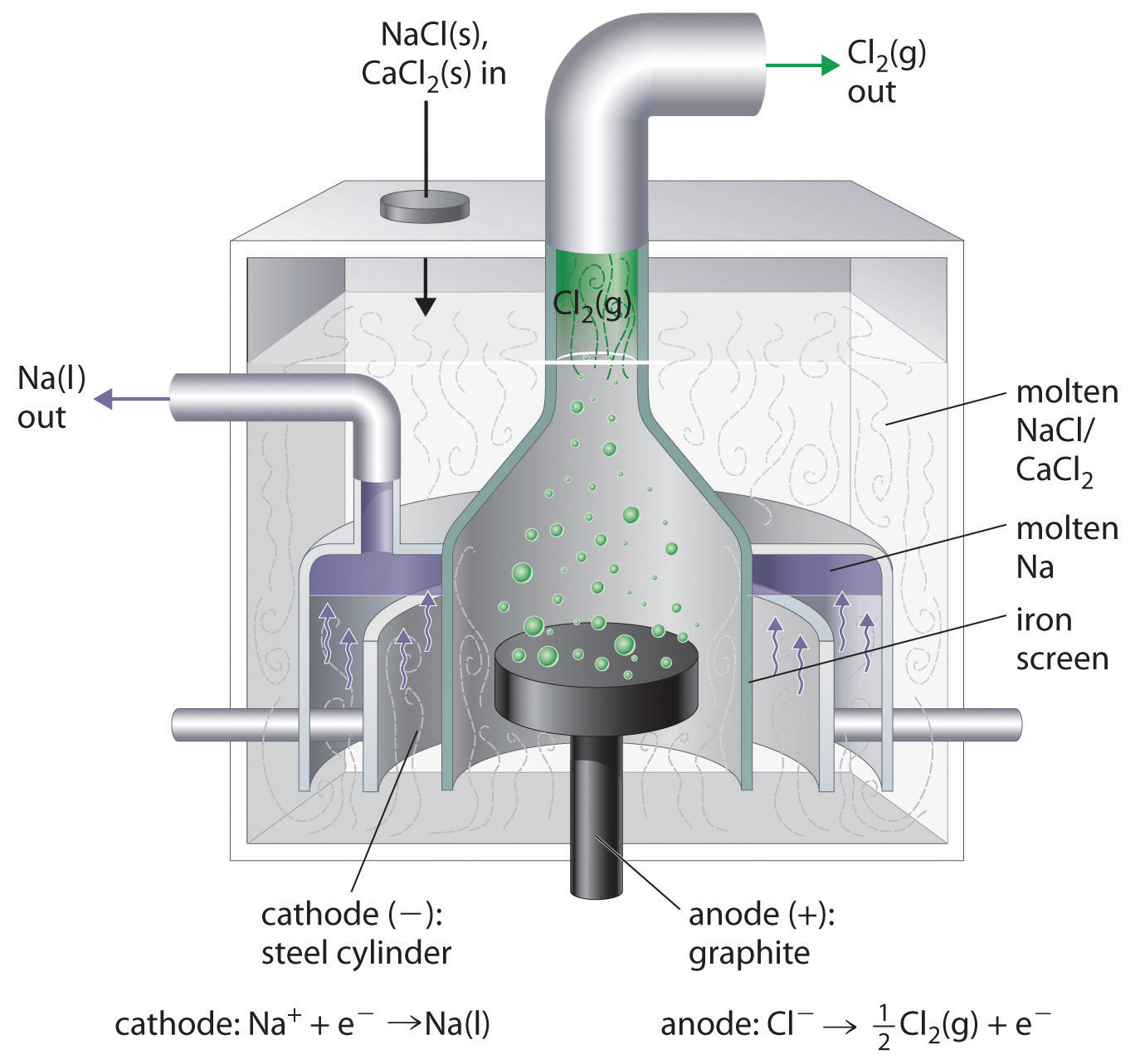
As can be seen from the cell in Figure \( {\PageIndex{2}}\), the chlorine gas formed at the anode and sodium metal formed at the cathode are kept separate by allowing Cl2 gas to bubble out of the cell and the sodium metal to collect above the cathode (since sodium's density is lower than that of molten NaCl it floats to the top of the molten sodium chloride).
Alkali metals can also be formed by chemical reduction. For example, potassium can be made by reducing potassium salts with Na, carbide, or carbon according to the following reactions.
\[ \ce{KCl + Na -> K + NaCl} \nonumber \]
\[ \ce{2 KX + CaC_2 \rightarrow 2 K + CaX_2 + C (X = F, Cl)} \nonumber \]
\[ \ce{K_2CO_3 + 2 C \rightarrow 2 K + 3 CO} \nonumber \]
Once formed, alkali metals are stored under an inert atmosphere or under hydrocarbon oil to prevent their reoxidation.
Properties
In metallic form, alkali metals possess a body centered cubic (BCC) structure and are silvery solids, as shown in Figure \( {\PageIndex{3}}\).

Like other metals, alkali metals are good conductors of heat and electricity, malleable, and ductile. However, compared to other metals alkali metals have a small number of valence electrons and relatively low effective nuclear charges. As a result the metallic bonds which hold solid and liquid alkali metals together are weaker than those in other metals and they melt and boil at lower temperatures, as illustrated by the melting and boiling points listed in Table \( {\PageIndex{1}}\). Further, as can be seen from the data in Table \( {\PageIndex{1}}\), both the melting and boiling points decrease down the alkali metal group. This decrease in melting and boiling points reflects a decrease in metallic bonding strength as the atomic size (and consequently average electron-nucleus distance) increases down the group.
| Substance | Melting Point (\(^{\circ}\) C) | Boiling Point (\(^{\circ}\) C) |
|---|---|---|
| Alkali Metal | ||
| Lithium, Li | 181 | 1347 |
| Sodium, Na | 98 | 883 |
| Potassium, K | 64 | 774 |
| Rubidium, Rb | 39 | 688 |
| Cesium, Cs | 28 | 678 |
| Francium | 27 | 677 |
| Non-alkali Metal | ||
| Magnesium | 649 | 1090 |
| Barium | 727 | 1845 |
| Titanium | 1660 | 3287 |
| Iron, Fe | 1538 | 2861 |
| Copper | 1083 | 2567 |
| Water | 0 | 100 |
| benzene | 6 | 80 |
The relatively low strength metallic bonding in the alkali metals is also reflected in their softness. The metals are so soft that they may be pressed into sheets and cut with an ordinary lab spatula. In fact, spatulas are commonly used to cut appropriate size portions of metal when they are used in the laboratory.
The atomic properties of alkali metals reflect the relatively high energy and large size of their ns valence orbitals. In consequence, they possess larger atomic radii and lower ionization energies than most metals, as may be seen from the data shown in Table \( {\PageIndex{2}}\).
| Substance |
Atomic radius (Angstroms) |
Ionization energy (kJ/mol) |
Pauling Electronegativity |
|---|---|---|---|
| Alkali Metal | |||
| Lithium, Li | 1.45 | 513 | 0.98 |
| Sodium, Na | 1.80 | 496 | 0.93 |
| Potassium, K | 2.20 | 419 | 0.82 |
| Rubidium, Rb | 2.35 | 403 | 0.82 |
| Cesium, Cs | 2.60 | 376 | 0.79 |
| Francium. Fr | not determined | 400 | 0.7 |
| Non-alkali Metal | |||
| Magnesium, Mg | 1.50 | 738 | 1.31 |
| Barium, Ba | 2.15 | 503 | 0.89 |
| Titanium, Ti | 1.40 | 658 | 1.54 |
| Iron, Fe | 1.40 | 759 | 1.83 |
| Copper, Cu | 1.35 | 745 | 1.90 |
| Boron, B | 0.85 | 801 | 2.04 |
| Nitrogen, N | 0.65 | 1402 | 3.04 |
As will be explained in the next section, the small ionization energies of the alkali metals is among the factors contributing to their extreme reactivity.
References
- The mineral to be electrolyzed must be capable of melting at suitably low temperatures, either on its own or when mixed with an electrolytically inert salt to lower its melting point.
- Alkali metal image credits: (a) Li: By Tomihahndorf at German Wikipedia - Transferred from de.Wikipedia to Commons., Public Domain, commons.wikimedia.org/w/inde...?curid=1744000; (b) Na: The original uploader was Dnn87 at English Wikipedia. / CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0); (c) K: Unknown author / CC BY (https://creativecommons.org/licenses/by/1.0); (d) Rb: Dnn87 / CC BY (https://creativecommons.org/licenses/by/3.0); and (e) Cs: Dnn87 Contact email: Dnn87yahoo.dk / CC BY-SA (https://creativecommons.org/licenses/by-sa/3.0)
- All physical and atomic property data for elements except atomic radii and the boiling and melting points of Ba and Fe are calculated from data in Emsley, J. The elements 2nd ed. Oxford University Press, 1991. The phase transition points for Ba and Fe were taken from https://www.rsc.org/periodic-table/element/56/barium and should be taken as tentative.
- Atomic radii are the empirical radii determined by John C. Slater as reported in Slater, J. C. J. Chem. Phys. 1964, 41, 3199-3204.


