23.3B: Magnesium
- Page ID
- 34548
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Grignard Reagents
Alkyl and aryl magnesium halides (Grignard reagents, \(\ce{RMgX}\)) are extremely well-known on account of their uses in synthetic chemistry. Over the past 100 years, Gringnard reagents had probably been the most widely used organometallic reagents. The general procedure for their preparation was discovered by Victor Grignard in 1900 and involved the direct reaction of magnesium with organohalides.
\[\ce{R-X + Mg → R-Mg-X, (X= Cl, Br, I)}\]
When the reaction is performed in diethyl ether or THF and in the absence of air and moisture, the compounds are reasonably stable although they need to be used immediately. Grignard reactions often start slowly. As is common for reactions involving solids and solution, initiation follows an induction period during which reactive magnesium becomes exposed to the organic reagents. After this induction period, the reactions can be highly exothermic.
Transmetallation is useful means of preparing pure Grignard reagents
\[ \ce{Mg + RHgBr -> Hg + RMgBr}\]
\[ \ce{Mg + R2Hg \rightarrow Hg + R2Mg}\]
Two-coordination at Mg in \(\ce{R2Mg}\) is observed only when the \(\ce{R}\) groups are sufficiently bulky, e.g. Mg{C(SiMe3)3}2. \(\ce{RMgX}\) are generally solvated and \(\ce{Mg}\) center is typically tetrahedral (e.g. EtMgBr.2Et2O; PhMgBr.2Et2O); Cp2Mg has a staggered sandwich structure.
The composition of ether solutions of Grignards was investigated by the Schlenk's who reported what is now called the Schlenk Equilibrium:
\[\ce{2 RMgX \rightleftharpoons MgX2 + MgR2} \label{schlenk}\]
The notation of \(\ce{RMgX}\) for Grigarnd reacgents is therefore an oversimplification of what truly exists in ether solutions. In diethyl ether, a tendency to form monomeric, dimeric and higher oligomeric species was found and was dependent on the halogen and organic substituents. Figure \(\PageIndex{1}\) indicates the percentage of \(\ce{RMgX}\) for a range of Grignard Reagents.
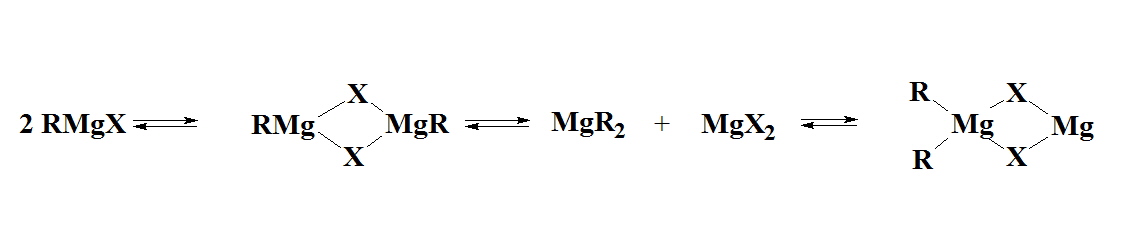
Figure \(\PageIndex{1}\): Composition of solutions of Grignard Reagents in diethyl ether solution at equilibrium. In diethyl ether, a tendency to form monomeric, dimeric and higher oligomeric species was found and was dependent on the halogen and organic substituents.
Solutions of Grignard reagent may contain several species, e.g. RMgX, R2Mg, MgX2, RMg(μ-X)2MgR, which are further complicated by solvation. The position of equilibrium between these species is markedly dependent on concentration, temperature and solvent; strongly donating solvents favor monomeric species in which they coordinate to the metal center.
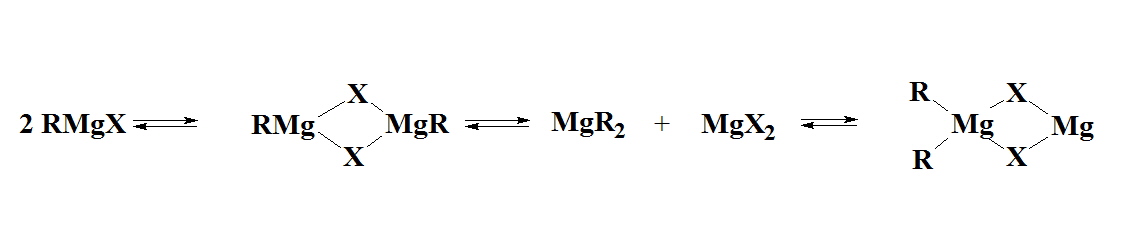
In tetrahydofuran (THF) the structures were found to be closer to monomeric but it was recognized that the solid state structures may be very different to what exists in solution. For example, when "EtMgCl" is isolated from a THF solution a tetramer was found where the Mg have coordination numbers higher than the expected 4.
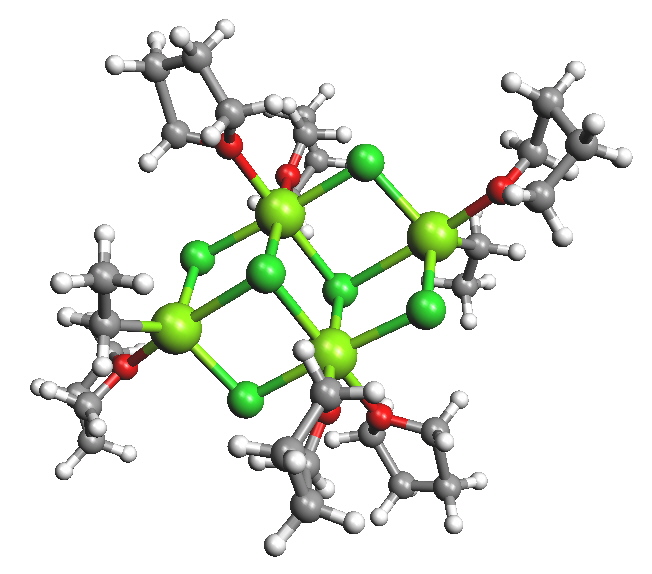
Figure \(\PageIndex{3}\): EtMgCl isolated from THF.
In the polymeric structures it is generally the halide, rather than the organic group that bridges between the magnesiums.
Reactions of Grignard Reagents
The most common application for the use of Grignard Reagents in Organic Chemistry is for alkylation of aldehydes and ketones, for example:
\[\ce{R1R2C=O + R3MgX → R1R2R3C-OMgX → R1R2R3C-OH}\]
and more generally:
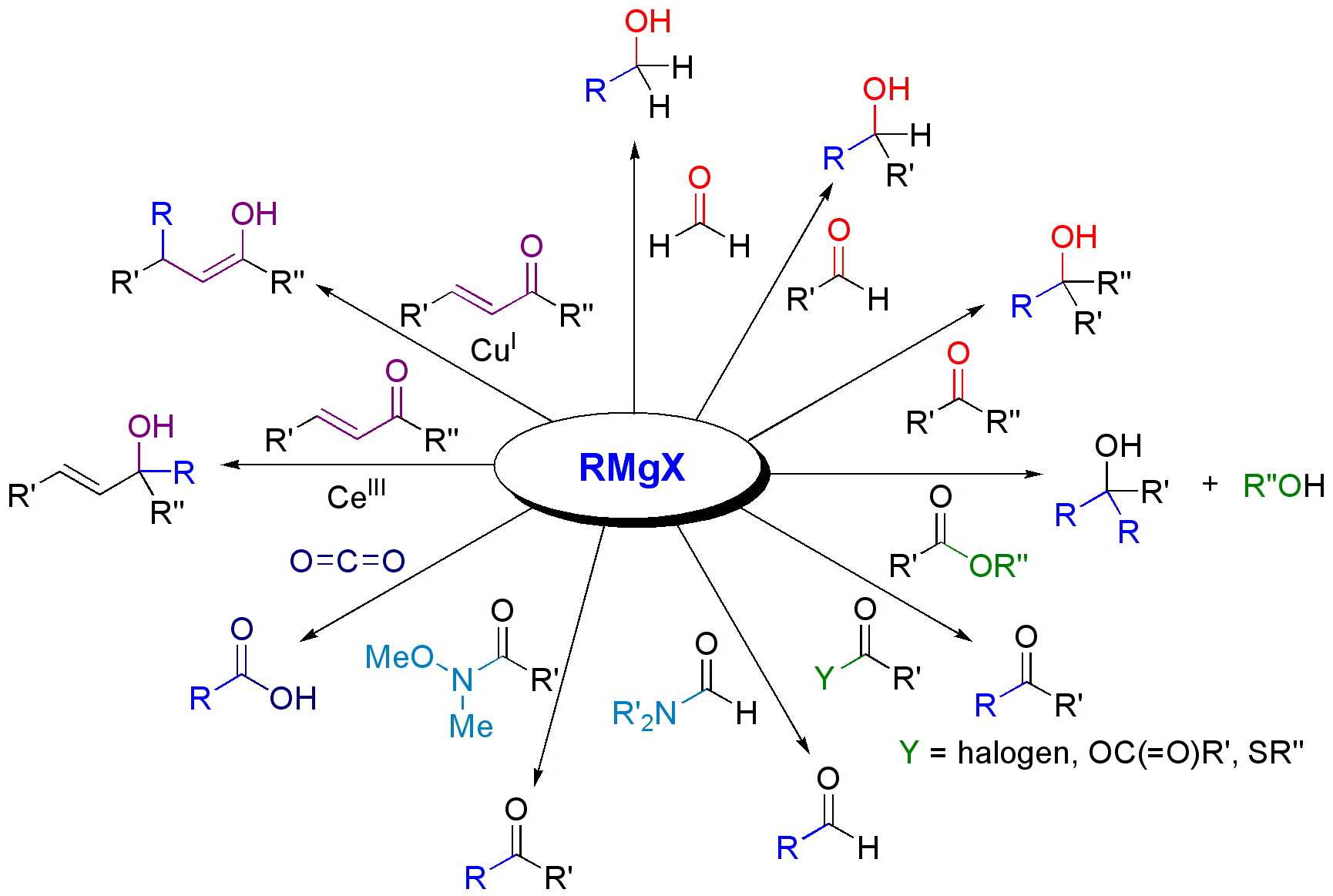
Figure \(\PageIndex{4}\): Reactions with carbonyls
Other reactions include carbon-carbon coupling which is often affected using a catalyst like transition metal halides.
\[\ce{2 ArMgX + MXn → Ar-Ar + MgX2 + MX_{n-2}}\]
and for cross-coupling reactions:
\[\ce{ArMgX + RCH=CHX ->[\text{5 mol% CoCl}_2] ArCH=CHR + MgBrX}\]
using, for example, 5 mol% CoCl2 as catalyst and to determine the difference in reactivity of the halides, a series of competitive reactions were performed: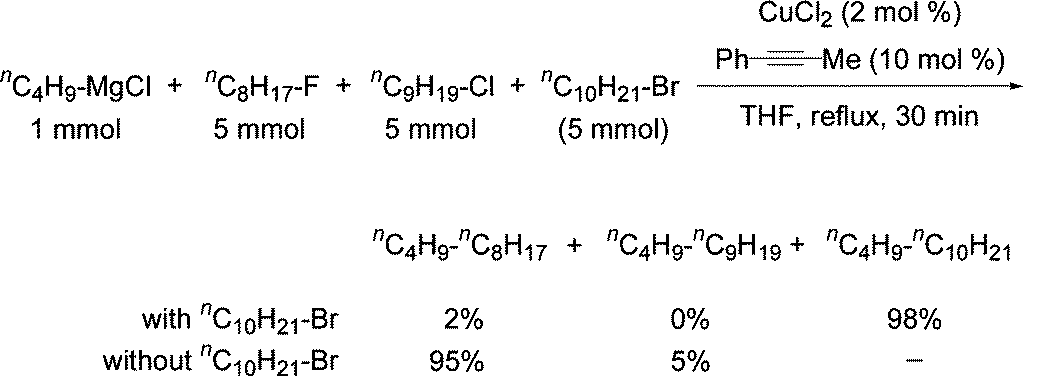
Figure \(\PageIndex{5}\): Cross-coupling Reactions with Grignard Reagents - 2008 review
Terao and Kambe reported that when they took a mixture of equimolar amounts of n-octyl fluoride, n-nonyl chloride, and n-decyl bromide and added to this CuCl2, 1-phenylpropyne, and a THF solution of n-ButylMgCl, then after the reaction was stirred for 30 min in THF at reflux, GC analysis of the resulting mixture indicated the selective formation of tetradecane in 98% yield along with 2% yield of dodecane. A similar reaction using only alkyl fluorides and chlorides gave dodecane and tridecane in 95% and 5% yields, respectively. These results indicated that the reactivity of alkyl halides was in the order:
\[\ce{bromide > fluoride > chloride}\]
| metal halide | amt, mol | amt of C6H5MgI, mol | yield of biphenyl, % |
|---|---|---|---|
| FeCl2 | 0.01 | 0.03 | 98 |
| CoBr2 | 0.01 | 0.03 | 98 |
| NiBr2 | 0.03 | 0.095 | 100 |
| RuCl3 | 0.0036 | 0.0108 | 99 |
| RhCl3 | 0.0036 | 0.013 | 97.5 |
| PdCl2 | 0.00566 | 0.0163 | 98 |
| OsCl3 | 0.00275 | 0.007 | 53 |
| IrCl3 | 0.003 | 0.01 | 28 |
A Grignard reaction that is a key step in an industrial production is shown below, where the target is Tamoxifen. Tamoxifen is an antagonist of the estrogen receptor in breast tissue and it is the standard endocrine (anti-estrogen) therapy for hormone-positive early breast cancer in post-menopausal women.
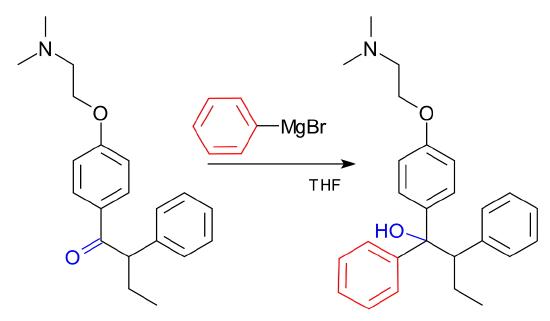
Figure \(\PageIndex{5}\): Tamoxifen synthesis using a Grignard Reagent
Problems
Q1
If a typical Grignard reagent exists as an equilibrium mixture of dialkylmagnesium and magnesium halide, give a method of isolating pure dialkyl magnesium. Your answer should be in the form of balanced chemical equations only.
Solution:
![]()
Treatment of equilibrium mixture with dioxane results in the precipitation of, say, MgCl2(dioxane) (if, X = Cl), leaving behind pure R2Mg in the solution.

Q2
The compound (Me3Si)2C(MgBr)2.nTHF is monomeric. Suggest a value of ‘n’ and propose a structure for this Grignard reagent.
Solution:

References
1. "Inorganic Chemistry" - C. Housecroft and A.G. Sharpe, Prentice Hall, 3rd Ed., Dec 2007, ISBN13: 978-0131755536, ISBN10: 0131755536, Chapter 17.
2. "Chemistry of the Elements", Greenwood and Earnshaw, Elsevier.
Contributors and Attributions
Prof. Robert J. Lancashire (The Department of Chemistry, University of the West Indies)

