8.2: What are the main group elements and why should anyone care about them?
- Page ID
- 199733
What are the main group elements?
The main group elements are those in the s and p blocks of the periodic table, as shown in Figure \(\PageIndex{1}\).
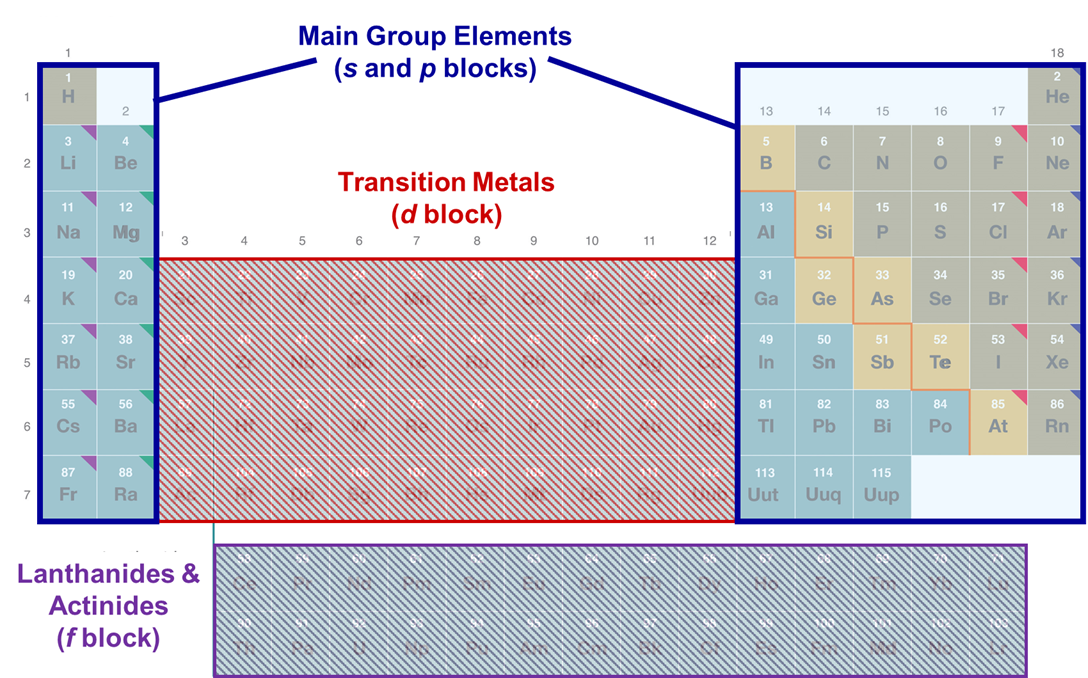
Why should anyone care about the main group elements?
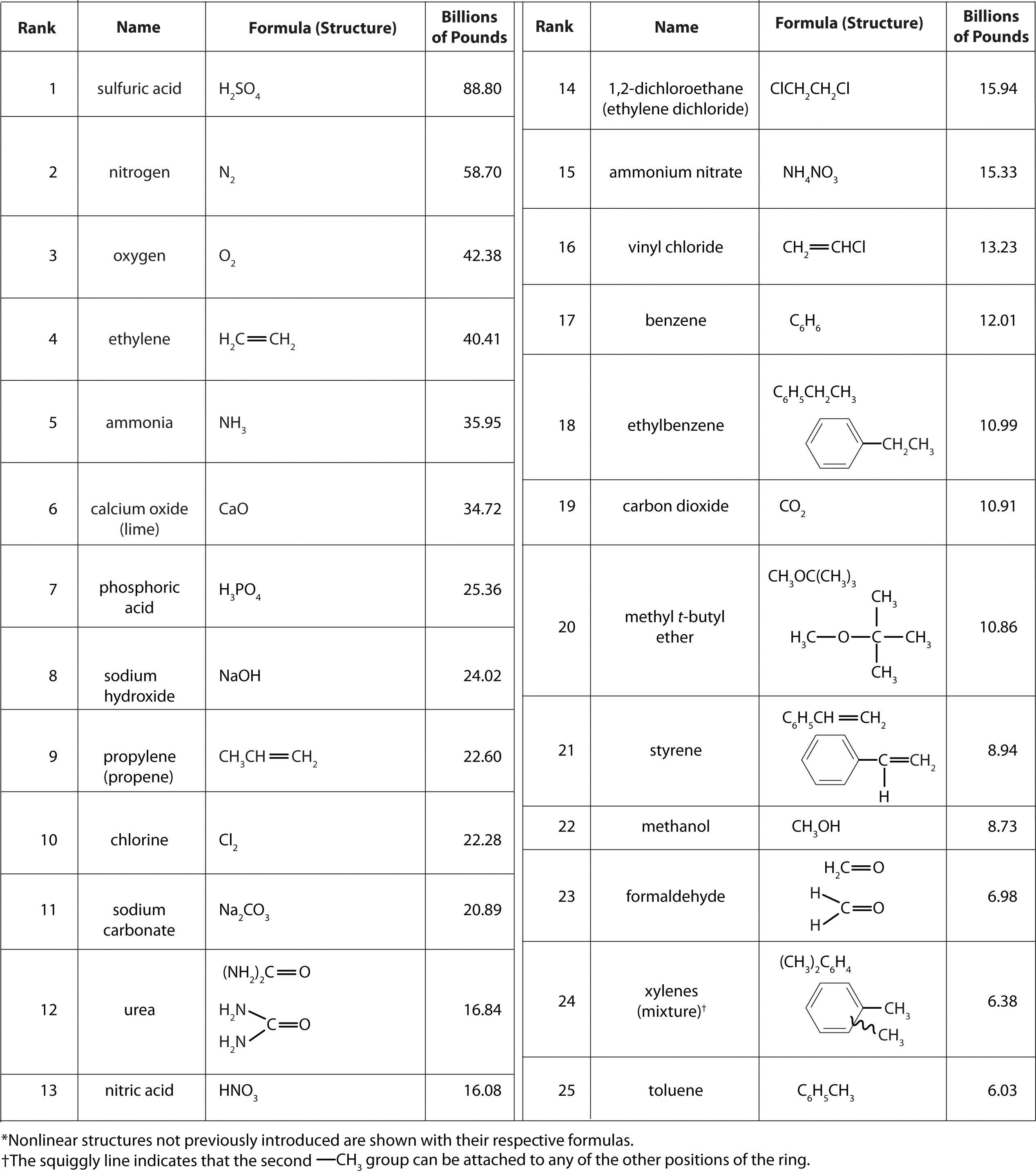
In addition to being interesting in their own right as fundamental constituents of ordinary matter with a rich and interesting chemistry, the main group elements are of tremendous societal importance. As shown in Table \(\PageIndex{1}\), thirteen of the top 25 chemicals produced in the United States are inorganic main group compounds.
Table \(\PageIndex{1}\). Top 25 industrial chemicals produced in the US in 2002.2
This chapter's approach to the descriptive chemistry of the main group elements
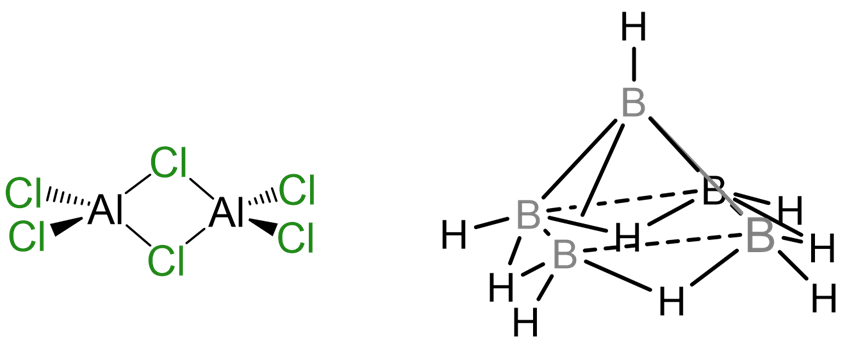
The aim of this chapter is to introduce you to the descriptive chemistry of the main group elements. In general descriptive chemistry involves presenting information about the chemistry of the elements, as opposed to describing more fundamental concepts, theories, and models. Nevertheless, in presenting this information no attempt will be made to be exhaustive; instead the focus will be on describing important or characteristic features of the chemistry. Moreover, as intimated by the structures shown in Scheme \(\sf{\PageIndex{I}}\), the bonds which hold main group compounds together do not always obey the rules developed in typical general and organic chemistry courses. Consequently, as opportunity presents itself, this description of the chemistry of the elements will also be used to enlarge your understanding of what types of chemical structures are possible or even common.
Scheme \(\sf{\PageIndex{I}}\). Chlorine atoms bridge Al centers in aluminum chloride dimer at left while the structure of pentaborane nonahydride shown at right includes four B-H-B bridge bonds arranged around the open face of a cluster of five B atoms held together by cluster bonds.
The elements will be considered by group after an introductory section that reviews several concepts that help to make sense of periodic trends in the descriptive chemistry of these elements.
References
1. Adapted from the periodic table at https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_Chemistry_-_The_Central_Science_(Brown_et_al.)/02._Atoms%2C_Molecules%2C_and_Ions/2.5%3A_The_Periodic_Table.
Contributor
Stephen Contakes, Westmont College
The unknown authors of Table \(\PageIndex{1}\) and the periodic table portion of Figure \(\PageIndex{1}\).
Unless otherwise noted, all line drawings on this page are by Stephen Contakes and licensed under a Creative Commons Attribution 4.0 International License.

