This page describes reactions of the halogens that do not fall under the other categories in other pages in this section. All the reactions described here are redox reactions.
Reactions with hydrogen
The following examples illustrate the decrease in reactivity of the halogens down Group 7.
Fluorine combines explosively with hydrogen even under cold, dark conditions, evolving hydrogen fluoride gas.
A mixture of chlorine and hydrogen explodes if exposed to sunlight or a flame, producing hydrogen chloride gas. This reaction can be controlled by lighting a jet of hydrogen and then lowering it into a gas jar of chlorine. The hydrogen burns at a slower, constant rate, and hydrogen chloride gas is formed as before.
Bromine vapor and hydrogen combine with a mild explosion when ignited. Hydrogen bromide gas is formed.
Iodine and hydrogen combine only partially even on constant heating. An equilibrium exists between the hydrogen and the iodine and hydrogen iodide gas.
Each of these reactions has an equation of the form:
\[ H_2 + X_2 \rightarrow 2HX \nonumber \]
A minor exception is made for iodine: the single arrow is replaced with a reversible sign.
Reactions with phosphorus
Care must be taken when analyzing the rates of these reactions; analogous reactions must be compared. For example, it is nonsensical to compare the rate at which phosphorus reacts with gaseous chlorine with the rate at which it reacts with liquid bromine. There is more contact between phosphorus and liquid bromine than between phosphorus and gaseous chlorine.
All halogens react with phosphorus to form, in the first instance, phosphorus(III) halides of the form PX3.
There are two common forms of phosphorus: white phosphorus (sometimes called yellow phosphorus) and red phosphorus. White phosphorus is more reactive than red phosphorus. This video on YouTube shows the reaction between red phosphorus and bromine. This is a violent reaction under cold conditions, and white phosphorus behaves even more dramatically.
When writing the equations for these reactions, it is important to remember that white phosphorus is molecular, consisting of P4 molecules, whereas red phosphorus is polymeric, indicated by the symbol P. The reaction for white phosphorus and bromine is as follows:
\[ P_4 + 6Br_2 \rightarrow 4PBr_3 \nonumber \]
The red phosphorus equation is shown below:
\[ 2P + 3Br_2 \rightarrow 2PBr_3 \nonumber \]
In excess chlorine or bromine, phosphorus reacts to form phosphorus(V) chloride or bromide. Most simply, using white phosphorus:
\[ P_4 + 10Cl_2 \rightarrow 4PCl_5 \nonumber \]
The reaction between phosphorus(III) chloride and phosphorus(V) chloride is reversible:
\[ PCl_3 + Cl_2 \rightleftharpoons PCl_5 \nonumber \]
An excess of chlorine pushes this equilibrium to the right. Phosphorus does not form a pentaiodide, in contrast; this is likely because five large iodine atoms cannot physically fit around the central phosphorus atom.
Reactions with sodium
All halogens react with sodium to produce sodium halides. A common reaction between hot sodium and chlorine gas produces a bright orange flame and white sodium chloride.
\[ 2Na + Cl_2 \rightarrow 2NaCl \nonumber \]
Hot sodium will also burn in bromine or iodine vapor to produce sodium bromide or sodium iodide. Each of these reactions produces an orange flame and a white solid.
Reactions with iron
With the exception of iodine, iron burns in halogen vapor, forming iron(III) halides. Iodine is less reactive, and produces iron(II) iodide.
Fluorine
Cold iron wool burns in cold fluorine to give iron(III) fluoride. Anhydrous iron(III) fluoride is described as either white or pale green. A standard inorganic chemistry textbook by Cotton and Wilkinson describes it as white. The reaction is given below:
\[ 2Fe + 3F_2 \rightarrow 2FeF_3 \nonumber \]
This is a rapid reaction, in which the iron burns and is oxidized to an iron(III) compound—in other words, from an oxidation state of zero in the elemental metal to an oxidation state of +3 in the iron(III) compound.
Chlorine
Chlorine gas in contact with hot iron forms iron(III) chloride. Anhydrous iron(III) chloride forms black crystals; any trace of water present in the apparatus or in the chlorine reacts with the crystals, turning them reddish-brown. The equation for this reaction is given below:
\[ 2Fe + 3Cl_2 \rightarrow 2FeCl_3 \nonumber \]
The iron is again oxidized from a state of zero to +3.
Bromine
Bromine vapor passed over hot iron triggers a similar, slightly less vigorous reaction, shown below; iron(III) bromide is produced. Anhydrous iron(III) bromide usually appears as a reddish-brown solid.
\[2Fe + 3Br_2 \rightarrow 2FeBr_3 \nonumber \]
In this reaction the iron is again oxidized to a +3 state.
Iodine
The reaction between hot iron and iodine vapor produces gray iron(II) iodide, and is much less vigorous. This reaction, the equation for which is given below, is difficult to carry out because the product is always contaminated with iodine.
\[ Fe + 2I_2 \rightarrow FeI_2 \nonumber \]
Iodine is only capable of oxidizing iron to the +2 oxidation state.
Reactions with solutions containing iron(II) ions
Only the reactions of chlorine, bromine, and iodine can be considered. Aqueous fluorine is very reactive with water. Chlorine and bromine are strong enough oxidizing agents to oxidize iron(II) ions to iron(III) ions. In the process, chlorine is reduced to chloride ions, bromine to bromide ions.
This process is easiest to visualize with ionic equations:

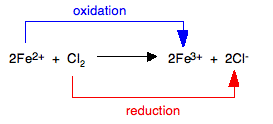
For the bromine equation, Br is substituted for Cl.
The pale green solution containing the iron(II) ions turns into a yellow or orange solution containing iron(III) ions. Iodine is not a strong enough oxidizing agent to oxidize iron(II) ions, so there is no reaction. In fact, the reverse reaction proceeds. Iron(III) ions are strong enough oxidizing agents to oxidize iodide ions to iodine as shown:
\[ 2Fe^{3+} + 2I^- \rightarrow 2Fe^{2+} + I_2 \nonumber \]
Reactions with sodium hydroxide solution
Once again, only chlorine, bromine, and iodine are considered.
The reaction of chlorine with cold sodium hydroxide solution
Chlorine and cold, dilute sodium hydroxide react as follows:
\[ 2NaOH + Cl_2 \rightarrow NaCl + NaClO + H_2O \nonumber \]
NaClO (sometimes written as NaOCl) symbolizes sodium chlorate(I). The traditional name for this compound is sodium hypochlorite; the solution on the product side of the equation is commonly sold as bleach.
Consider this reaction in terms of oxidation states. Chlorine displays an obvious state change from its elemental form to ionic compounds. The oxidation numbers for each element are shown below:

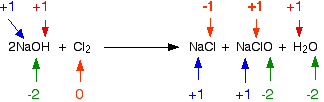
Chlorine is the only element that changes oxidation state—it is both oxidized and reduced. One atom is reduced because its oxidation state has decreased; the other is oxidized. This is a good example of a disproportionation reaction, a reaction in which a single substance is both oxidized and reduced.
The reaction of chlorine with hot sodium hydroxide solution
Chlorine reacts with hot, concentrated sodium hydroxide as follows:
\[ 6NaOH + 3Cl_2 \rightarrow 5NaCl + NaClO_3 + 3H_2O \nonumber \]
The product formed is sodium chlorate(V) - NaClO3. As before, the oxidation states of each element are calculated. Once again, the only change is in chlorine, from 0 in the chlorine molecules on the reactant side to -1 (in the NaCl) and +5 (in the NaClO3). This is another example of a disproportionation reaction.
Balancing equations for these reactions
The first equation is simple to balance. The second one is more difficult; oxidation states are used to derive it.
The two main products of the reaction are NaCl and NaCIO3, so the reaction can be tentatively written as follows:
\[ NaOH + Cl_2 \rightarrow NaCl + NaClO_3 + ? \nonumber \]
In its conversion to NaCl, the oxidation state of the chlorine decreases from 0 to -1. When converted to NaClO3, it increases from 0 to +5. The positive and negative oxidation state changes must cancel out, so for every NaClO3 formed, there must be 5 NaCl:
\[ NaOH + Cl_2 \rightarrow 5NaCl + NaClO_3 + ? \nonumber \]
Now it is a simple task to balance the sodium and the chlorine atoms, after which there are enough hydrogen and oxygen atoms to make 3H2O.
The reactions involving bromine and iodine
These are essentially similar to that of chlorine; the difference lies in the reaction temperatures. The tendency to form the ion with the halogen in the +5 oxidation state increases rapidly down the group.
Bromine and sodium hydroxide solution
For bromine, the formation of the sodium bromate(V) happens at around room temperature. Sodium bromate(I) must be formed at about 0°C.
Iodine and sodium hydroxide solution
In this case, sodium iodate(V) is formed at any temperature. Cotton and Wilkinson (Advanced Inorganic Chemistry 3rd edition page 477) say that the iodate(I) ion is unknown in solution.




