6.3.8: High Charge-to-Size Ratio Metal Ions Act as Brønsted Acids in Water
- Page ID
- 157379
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Metal Ions Act as Acids by Polarizing Bound Water Molecules Called Aqua Ligands
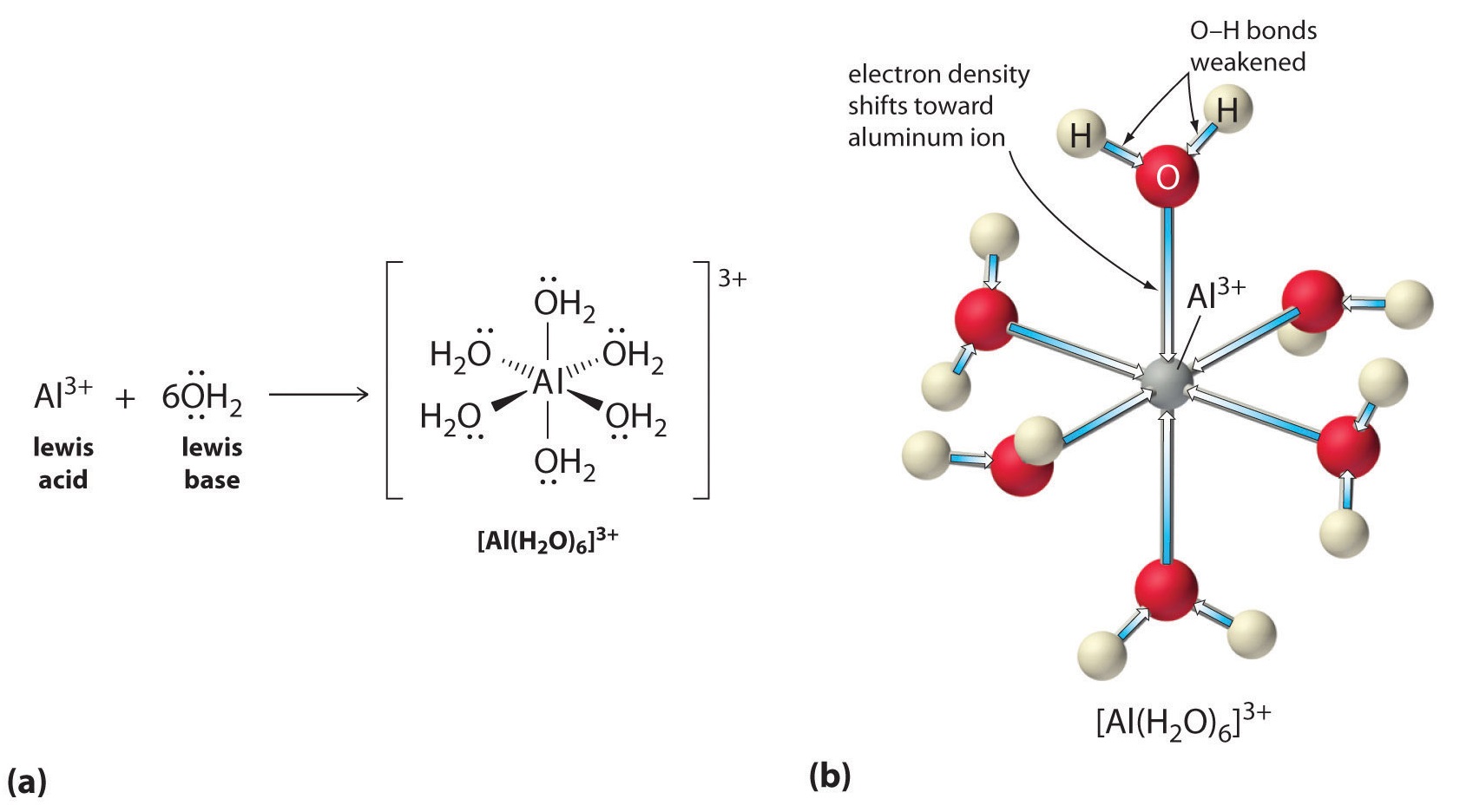
Aqueous solutions of simple salts of metal ions can also be acidic, even though a metal ion cannot donate a proton directly to water to produce \(H_3O^+\). Instead, a metal ion can act as a Lewis acid and interact with water, a Lewis base, by coordinating to a lone pair of electrons on the oxygen atom to form a hydrated metal ion.
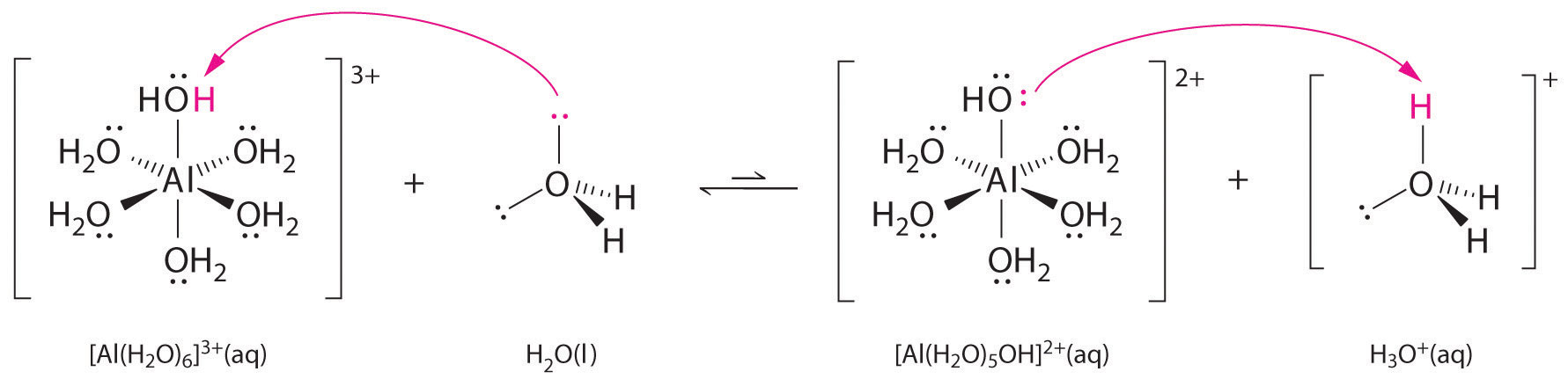
A water molecule coordinated to a metal ion is more acidic than a free water molecule for two reasons. First, repulsive electrostatic interactions between the positively charged metal ion and the partially positively charged hydrogen atoms of the coordinated water molecule make it easier for the coordinated water to lose a proton.
Second, the positive charge on the \(Al^{3+}\) ion attracts electron density from the oxygen atoms of the water molecules, which decreases the electron density in the \(\ce{O–H}\) bonds, as shown in Figure \(\PageIndex{5b}\). With less electron density between the \(O\) atoms and the H atoms, the \(\ce{O–H}\) bonds are weaker than in a free \(H_2O\) molecule, making it easier to lose an \(H^+\) ion. This is shown schematically in Figure \(\PageIndex{1}\).
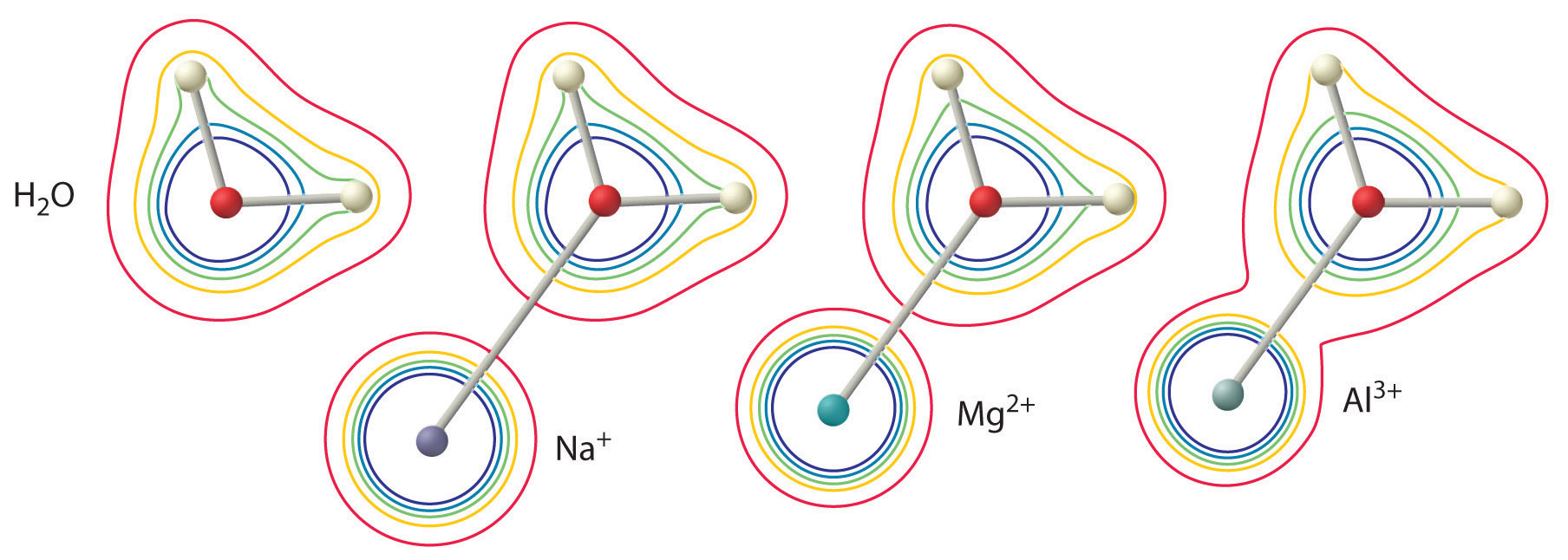
The acidity of a given metal ion largely depends on its charge to size ratio and electronegativity, although in some cases hardness and ligand field effects also play a role.
The magnitude of this effect depends on the following factors, of which the first two are generally considered the most important (Figure \(\PageIndex{2}\)):
The charge on the metal ion
A divalent ion (\(\ce{M^{2+}}\)) has approximately twice as strong an effect on the electron density in a coordinated water molecule as a monovalent ion (\(\ce{M^{+}}\)) of the same radius.
The radius of the metal ion
For metal ions with the same charge, the smaller the ion, the shorter the internuclear distance to the oxygen atom of the water molecule and the greater the effect of the metal on the electron density distribution in the water molecule.
The first two of these factors explain why most alkali metal cations exhibit little acidity while aqueous solutions of small, highly charged metal ions, such as \(Al^{3+}\) and \(Fe^{3+}\), are acidic:
\[\ce{[Al(H2O)6]^{3+}(aq) <=> [Al(H2O)5(OH)]^{2+}(aq) + H^{+}(aq)} \label{16.36} \]
The \(\ce{[Al(H2O)6]^{3+}}\) ion has a \(pK_a\) of 5.0, making it almost as strong an acid as acetic acid. Because of the two factors described previously, the most important parameters for predicting the effect of a metal ion on the acidity of coordinated water molecules are the charge and ionic radius of the metal ion.
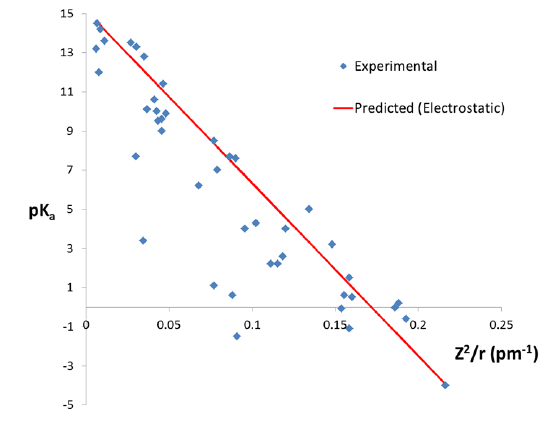
A simple empirical equation for predicting the pKa of metal ions in water has been proposed by Wulfsberg:1
\[pK_a = 15.14 - 88.16~pm \left( \frac{Z^2}{r} \right) \nonumber \]
where \(Z\) is the charge on the metal ion and \(r\) its radius. As can be seen from figure \(\PageIndex{3}\), in general the acidity of metal ions increases with \( \frac{Z^2}{r}) \). Nevertheless a number of ions have considerably lower \(pK_a\) values than predicted from this correlation, suggesting that the acidity of metal cations cannot be predicted using a simple electrostatic model alone.
Although the charge-to-size ratio is the simplest and most powerful predictor of metal ion acidity in water, three additional factors also can play a role:
Electronegativity
All other things being equal, more electronegative elements are better able to withdraw electron density from a bound water ligand and consequently better at enhancing the ability of that water molecule to lose a hydrogen ion. The electrostatic model of ion acidity can be extended to account for electronegativity effects but only needs to be done so for metals with Pauling electronegativities greater than 1.5. The empirical relationship that has been proposed to account for the effect of electronegativity is:
\[pK_a = 15.14 - 88.16 \left( \frac{Z^2}{r} + 0.096( \chi - 1.50) \right) \nonumber \]
where \(r\) is the ion radius in pm and \( \chi \) is its Pauling electronegativity. As can be seen from figure \(\PageIndex{4}\), the electronegativity term accounts for some of the deviations in metal ion acidity predicted from charge and size effects alone.
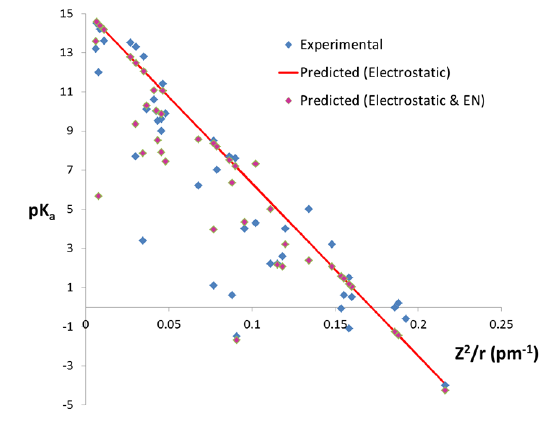
However, there are still large deviations between the predicted and observed pKa for a number of ions. In particular, the modified electrostatic model overestimates the pKa of Al3+ and Sn4+ and underestimates the pKa of Hg2+, Sn2+, and Tl3+. While the exact reasons for these discrepancies is not entirely clear, at least some are thought to arise from the impact of the fourth factor that determines metal ion acidity.
Hardness and Softness
Cation hardness or softness is assessed according to Pearson's Hard-Soft Acid Base Principle (HSABP). In general, soft cations are more acidic than hard cations of the same charge and radius, as may be seen from the examples in Table \(\PageIndex{1}\). The greater than expected acidity of softer cations is thought to reflect the importance of covalent contributions to the metal-water bond.
| Cation | Classification | Radius (pm) | \(pK_a1\) |
|---|---|---|---|
| \(K^+\) | hard | 1.33 | 14 |
| \(Ag^+\) | soft | 1.26 | 10 |
| \(Mg^{2+}\) | hard | 0.65 | 12.2 |
| \(Cu^{2+}\) | soft | 0.69 | 7.3 |
| \(Ca^{2+}\) | hard | 0.99 | 12.6 |
| \(Cd^{2+}\) | soft | 0.97 | 9.0 |
| \(Sr^{2+}\) | hard | 1.13 | 13.1 |
| \(Hg^{2+}\) | soft | 1.10 | 3.6 |
Ligand field effects
Ligand field effects involve bonding and antibonding interactions between the d orbitals on a transition metal and ligand orbitals. The importance of ligand field interactions on cation acidity is not well established, but ligand field interactions might influence the acidity of a hydrated metal in two cases: (a) when the ligand field stabilization of the aqua complex (metal with \(\ce{H_2O}\) or \(\ce{OH^{-}}\) bound) is greater or less than that in its conjugate base and (b) in complexes which undergo Jahn-Teller distortions that alter the metal's ability to polarize the OH bond.
References
- Wulfsberg, G. Principles of Descriptive Inorganic Chemistry University Science Books, 1991, pp. 28-30.
- (a) Gutmann, V. Allg. Prakt. Chem. 1970, 21, 116. (b) Gutmann, V. Fortschr. Chem. Forsch. 1972, 27, 59.
- Burgess, J. Metal Ions in Solution Ellis Horwood, 1978, pg. 268.

