10.3: Compounds of Chlorine
- Page ID
- 212674
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Comparison to fluorine
To appreciate the chemistry of chlorine in comparison to that of fluorine it is necessary to appreciate the differences and trends between the elements. As may be seen in Table \(\PageIndex{1}\), chloride is significantly larger than fluorine. In addition while chlorine is an electronegative element its electronegativity is significantly less than that of fluorine, resulting in less polar bonding.
| Element | Ionic radius (Å) | Covalent radius (Å) | van der Waal radius (Å) | Electronegativity |
|---|---|---|---|---|
| Fluorine | 1.33 | 0.64 | 1.47 | -4.1 |
| Chlorine | 1.81 | 0.99 | 1.75 | -2.9 |
The X-Cl bond is an electron pair covalent bond with a highly polar nature. In this regard, chlorine is similar to fluorine. However, there are two key features with regard to chlorine’s bonding that differentiates it from fluorine.
- Unlike fluorine, chlorine can form multiple covalent bonds, e.g., ClO4- and ClF3.
- Unlike fluorine, chlorine can form π-bonds with oxygen, i.e., Cl=O.
The chloride ion (Cl-) forms salts with ionic lattices (e.g., NaCl) but also forms a wide range of complexes, e.g., [Fe(H2O)5Cl]2+ and [RhCl6]3-. Chloride also acts as a bridging ligand in which one, two or three chlorides can bridge two metal centers (Figure \(\PageIndex{1}\)).
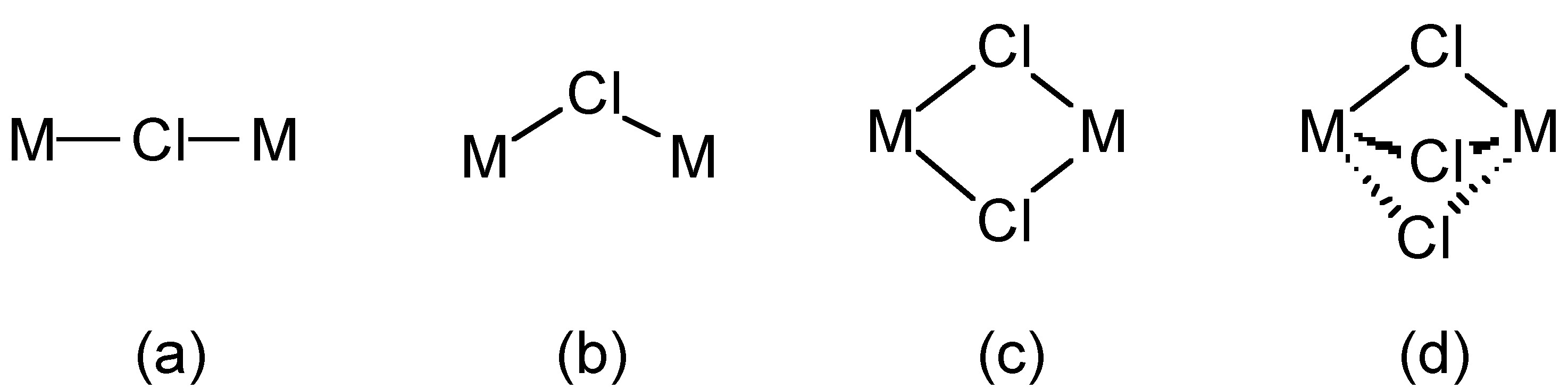
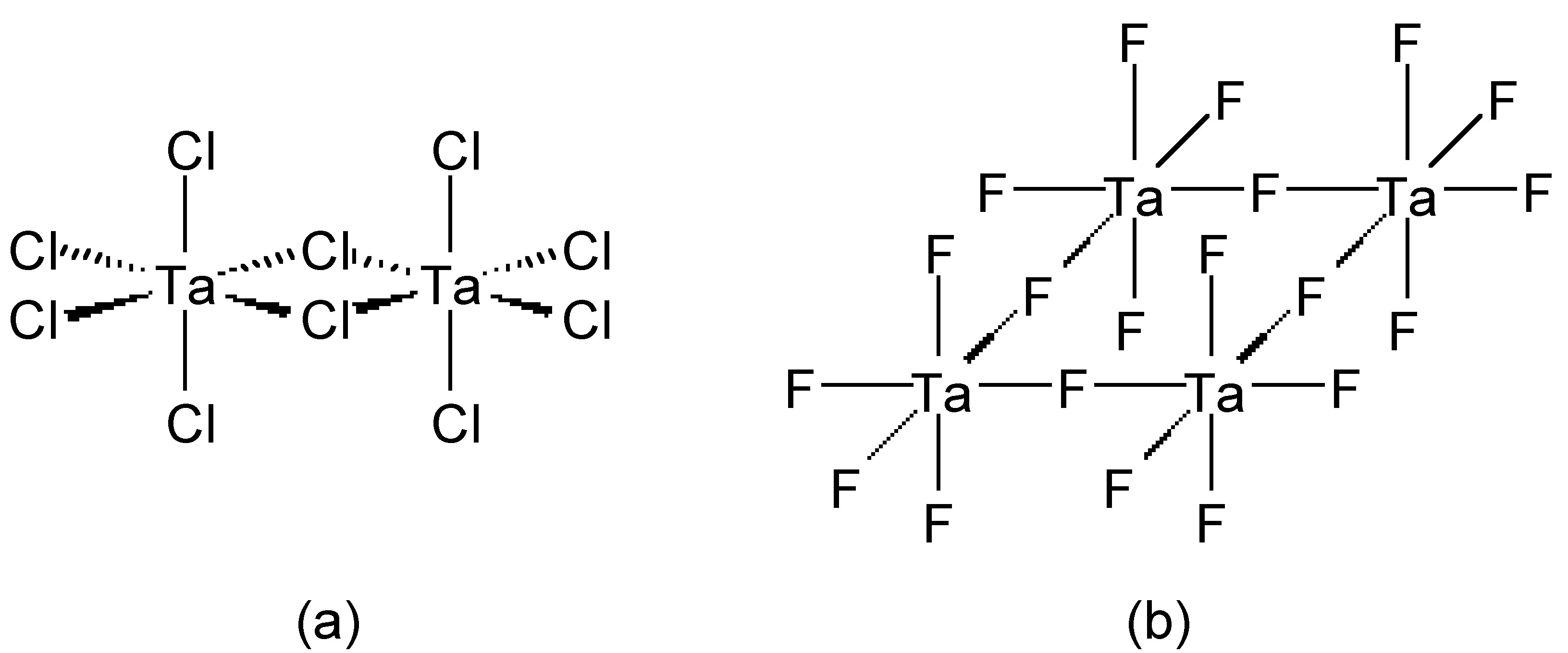
Chloride (and bromide) bridges are usually bent, whereas fluoride bridges can be either linear or bent. As an example, BeF2 and BeCl2 are isostructural, consisting of infinite chains with bent bridges (Figure \(\PageIndex{2}\)). In contrast, transition metal pentahalides show different structures depending on the identity of the halide. This, TaCl5 dimerizes with bent bridges (Figure \(\PageIndex{3}\)a), while TaF5 forms a cyclic tetramer with linear fluoride bridges (Figure \(\PageIndex{3}\)b).
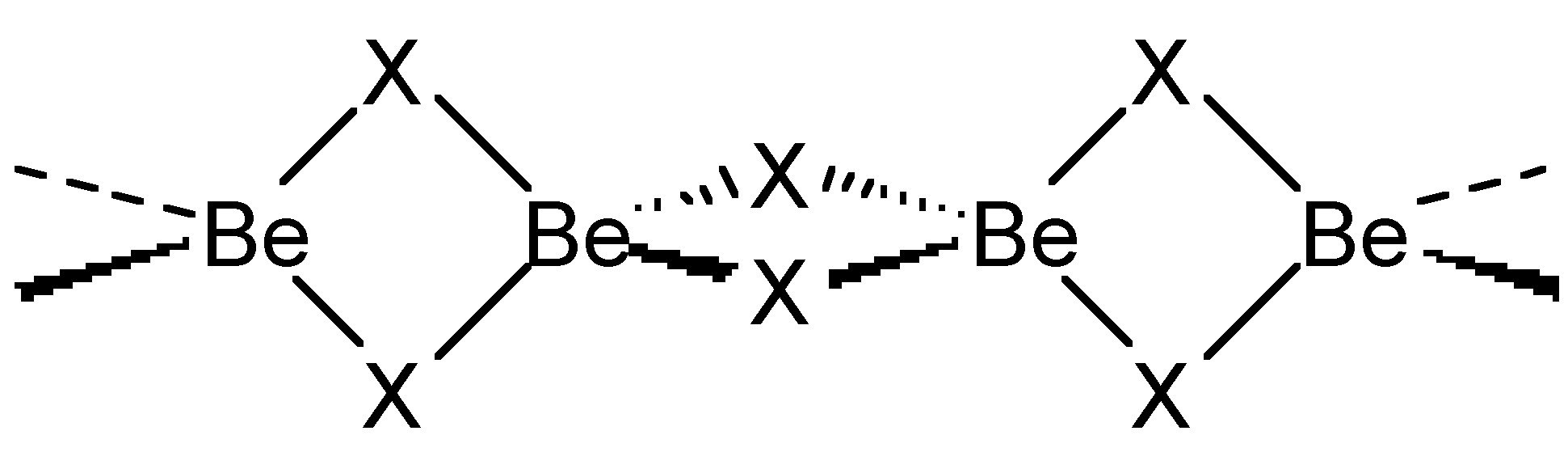
The halide bridge
The bridging halide bonds can be described by both Lewis and molecular orbital (MO) theory. In a simple picture, the lone pair of a terminal halide can be thought to act as a Lewis base donor ligand to the second Lewis acidic metal center. Indeed some bridging halides are asymmetric consistent with this view; however, the symmetrical ones can be described by a resonance form. From a molecular orbital point of view, the bridging halide is represented by a combination of two metal centered orbitals with two halogen orbitals.
Hydrogen chloride
Hydrogen chloride (HCl) is prepared by the reaction of concentrated sulfuric acid (H2SO4) with either NaCl or concentrated HCl solution.
Hydrogen chloride is a polar molecule with a dipole of 1.08 D. However, the lower polarity as compared to that of hydrogen fluoride (1.91 D) is consistent with the physical and chemical properties. Hydrogen chloride is a gas at room temperature (Mp = -114.25 °C, Bp = -85.09 °C), and its low boiling point is consistent with weak hydrogen bonding in the liquid state. While self-ionization, (10.3.1), is very small, liquid HCl dissolves some inorganic compounds to give conducting solutions, (10.3.2).
\[ \rm 3 HCl \rightleftharpoons H_2Cl^+ + HCl_2^-\]
\[ \rm R_3N + 2 HCl \rightleftharpoons R_3NH^+ + HCl_2^-\]
Hydrogen chloride is soluble (and reacts) in water, (10.3.3). The pKa of the reaction (-7.0) is larger than observed for fluorine (3.2) and as such HCl is a stronger acid than HF.
\[ \rm HCl + H_2O \rightleftharpoons H_3O^+ + Cl^- \]
Oxides of Chlorine
Chlorine forms a series of oxides (Table \(\PageIndex{2}\)) in which the chlorine has the formal oxidation states +1, +4, +6, and +7. The physical properties of the oxides are summarized in Table \(\PageIndex{2}\). While, the oxides of chlorine are not very stable (in fact several are shock sensitive and are prone to explode) the conjugate oxyacids are stable.
| Compound | Mp (°C) | Bp (°C) |
|---|---|---|
| Cl2O | -116 | 4 |
| ClO2 | -5.9 | 10 |
| Cl2O4 | -117 | 44.5 |
| Cl2O6 | 3.5 | unstable |
| Cl2O7 | -91.5 | 82 |
Dichlorine monoxide (Cl2O, Figure \(\PageIndex{4}\)a) is a yellowish-red gas that is prepared by the reaction of chlorine with mercury oxide, (10.3.4), or with a solution of chlorine in CCl4.
\[ \rm 2 Cl_2 + 2 HgO \rightarrow HgCl_2 \cdot HgO + Cl_2O\]

When heated or subject to a spark, Cl2O explodes to Cl2 and O2. Dichlorine monoxide reacts with water to form an orange-yellow solution of hypochlorous acid, (10.3.5).
\[ \rm H_2O_{(g)} + Cl_2O_{(g)} \rightleftharpoons 2 HOCl_{(g)} \]
Chlorine dioxide (ClO2) is a yellowish gas at room temperature and is commonly used in industry as an oxidizing agent. The best synthesis of ClO2 involves the reduction of potassium chlorate (KClO3) by oxalic acid at 90 °C, since the CO2 formed acts as a diluent for the highly explosive ClO2. On an industrial scale ClO2 is made by the exothermic reaction of sodium chlorate with SO2 in sulfuric acid, (10.3.6). The photolysis of ClO2 yields a dark brown solid with the formula Cl2O3; however, its facile explosive decomposition precludes study.
\[ \rm 2 NaClO_3 + SO_2 + H_2SO_4 \rightarrow 2 ClO_2 + 2 NaHSO_4\]
The structure of ClO2 (Figure \(\PageIndex{4}\)b) is equivalent to SO2 with one extra electron, resulting in a paramagnetic unpaired electron species. Unusually, despite the unpaired electron configuration, ClO2 shows no tendency to dimerize. This is unlike the analogous NO2 molecule.
Dichlorine tetraoxide (Cl2O4) is commonly called chlorine perchlorate as a consequence of its structure (Figure \(\PageIndex{4}\)c). Dichlorine hexaoxide (Cl2O6) is an unstable red oil that has the ionic structure in the solid state: [ClO2]+[ClO4]-.
Dichlorine heptoxide (Cl2O7) is a relatively stable oil, that is prepared by the dehydration of perchloric acid at -10 °C, (10.3.7), followed by vacuum distillation. The structure of Cl2O7 (Figure \(\PageIndex{4}\)d) has been determined by gas phase electron diffraction.
\[ \rm HClO_4 \xrightarrow[-H_2O]{+ P_4O_5} Cl_2O_7\]
The reaction of Cl2O7 with alcohols and amines yields alkyl perchlorates (ROClO3) and amine perchlorates (R2NClO3), respectively.
Fluorides of chlorine
Given the isolobal relationship between the halogens it is not surprising that the mixed dihalogens can be prepared, e.g., ClF, ICl, and BrCl. Chlorine fluoride is a highly reactive gas (Bp = -100.1 °C) that is a powerful fluorinating agent, and is prepared by the oxidation of chlorine by chlorine trifluoride, (10.3.8).
\[ \rm Cl_2 + ClF_3 \rightarrow 3 ClF\]
The higher electronegativity of fluorine as compared to chlorine (Table \(\PageIndex{1}\)), and the ability of chlorine to form more than one bond, means that higher fluorides of chlorine are also known, i.e., ClF3 and ClF5. Chlorine trifluoride (CF3, Bp = 11.75 °C) is a useful fluorinating agent, that is prepared by the high temperature reaction of elemental chlorine and fluorine, is a useful fluorinating age. The gaseous pentafluoride (ClF5, Bp = -31.1 °C) is prepared by the reaction of potassium chloride with fluorine, (10.3.10).
\[ \rm Cl_2 + 3 F_2 \xrightarrow{200 °C} 2 ClF_3\]
\[ \rm KCl + 3 F_2 \xrightarrow{200 °C} ClF_5 + KF\]
The structure of ClF3 is T-shaped with two lone pairs on chlorine (Figure \(\PageIndex{5}\)a), while that of ClF5 is square pyramidal with a single lone pair on chlorine (Figure \(\PageIndex{5}\)b).

In general the halogen fluorides are very reactive; explosive reactions occur with organic compounds. They are all powerful fluorinating agents when diluted with nitrogen, and the order of reactivity follows:
\[ \rm ClF_3 > BrF_3 > BrF_5 > IF_7 > ClF > IF_5 > BrF\]
Like most halogen fluorides, ClF, ClF3 and ClF5 all react with strong bases (e.g., alkali metal fluorides) to form anions, (10.3.12) and (10.3.13), and strong acids (e.g., AsF5 and SbF5) to form cations, (10.3.14), (10.3.15), and (10.3.16).
\[ \rm ClF + CsF \rightarro Cs^+ + ClF_2^-\]
\[ \rm ClF_3 + CsF \rightarrow Cs^+ + ClF_4^- \]
\[ \rm 2 ClF + AsF_5 \rightarrow FCl_2^+ + AsF_6^-\]
\[ \rm ClF_3 + AsF_5 \rightarrow ClF_2^+ + AsF_6^-\]
\[ \rm ClF_5 + SbF_5 \rightarrow ClF_4^+ + SbF_6^-\]


