6.10: Boron Compounds with Nitrogen Donors
- Page ID
- 212886
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The “B-N” unit is isoelectronic (3 + 5 valence electrons) to the “C-C” unit (4 + 4 valence electrons). The two moieties are also isolobal, and as such there are many of the compound types formed by carbon have analogous derivatives in the chemistry of boron-nitrogen.
Lewis acid-base addition compounds
Boron compounds, BX3, are strong Lewis acids and as such form stable addition compounds with Lewis bases, in particular those with nitrogen donor ligands.
\[ \text{BF}_3 \text{ + NMe}_3 \rightarrow \text{F}_3\text{B-NMe}_3\]
In principle these Lewis acid-base complexes should be similar to their isolobal hydrocarbon analogs, however, whereas the dipole in ethane is zero (by symmetry) the dipole in H3NBH3 is 5.2 D as a consequence of the difference in the Pauling electronegativities (i.e., B = 2.04 and N = 3.04). It is this dipole that generally differentiates the B-N compounds from their C-C analogs.
Homolytic cleavage of the C-C bond in ethane will yield two neutral methyl radicals, (6.7.2). In contrast, heterolytic cleavage will result in the formation of two charged species, (6.7.3). Thus, the products either have a net spin, (6.7.2), or a net charge, (6.7.3). By contrast, cleavage of the B-N bond in H3N-BH3 either yields products with both spin and charge, (6.7.5), or neither, (6.7.4). Heterolytic cleavage of the B-N bond yields neutral compounds, (6.7.4), while hemolytic cleavage results in the formation of radical ions, (6.7.5).
\[\text{H}_3\text{C-CH}_3 \rightarrow \cdot\text{CH}_3 \text{ + } \cdot\text{CH}_3 \]
\[\text{H}_3\text{C-CH}_3 \rightarrow \text{CH}^+_3 \text{ + } \cdot\text{CH}^-_3\]
\[\text{H}_3\text{N-BH}_3 \rightarrow \text{NH}_3 \text{ + BH}_3 \]
\[\text{H}_3\text{N-BH}_3 \rightarrow \text{NH}^+_3\cdot \text{ + BH}^-_3\cdot\]
The difference in bond strength between H3N-BH3 and ethane is reflected in the difference in bond lengths (Table \(\PageIndex{1}\)).
| Compound | Bond length (Å) | Bond strength (kcal/mol) |
| H3C-CH3 | 1.533 | 89 |
| H3N-BH3 | 1.658 | 31 |
Aminoboranes
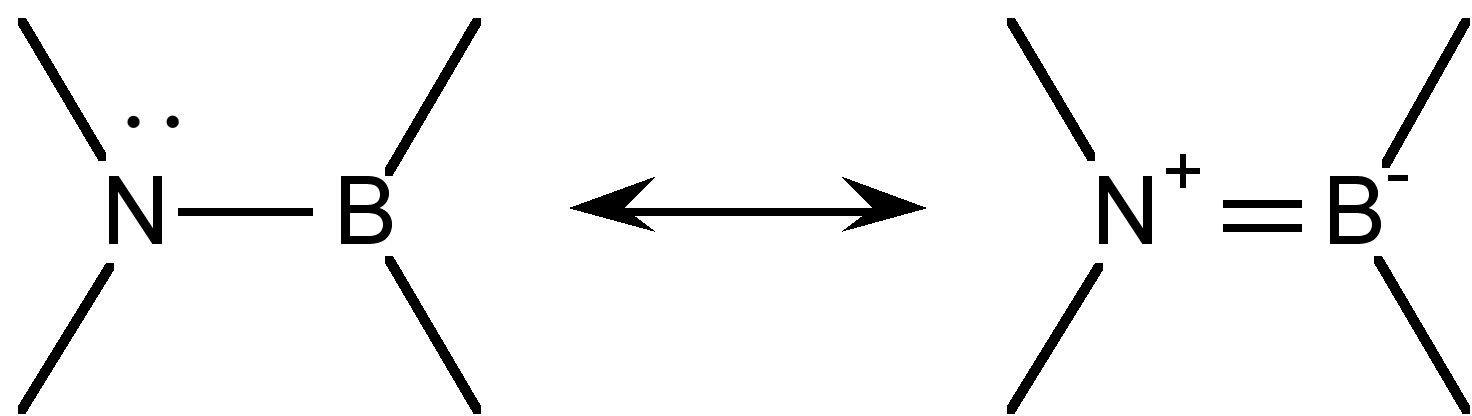
The group R’2N-BR2 is isoelectronic and isolobal to the olefin sub-unit R’2C=CR2, and there is even appreciable π-bonding character (Figure \(\PageIndex{1}\)). A measure of the multiple bond character can be seen from a comparison of the calculated B-N bond in H2NBN2 (1.391 Å) as compared to a typical olefin (1.33 Å). It is interesting that a consideration of the possible resonance form (Figure \(\PageIndex{1}\)) suggest the dipole in the σ-bond is in the opposite direction of that of the π-bond.
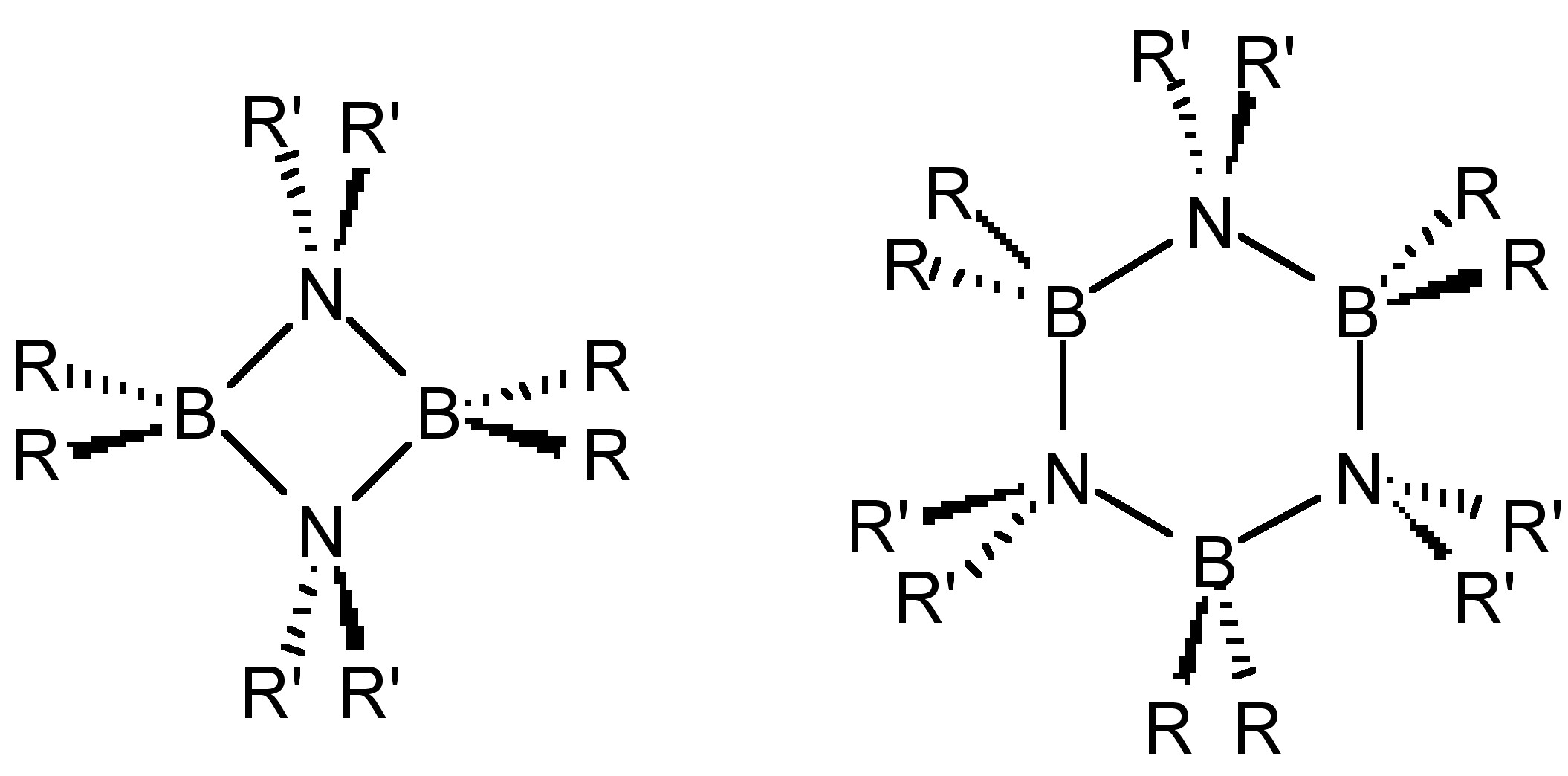
Unlike olefins, borazines oligomerize to form dimmers and trimers (Figure \(\PageIndex{2}\)) in the absence of significant steric hindrance. Analogous structures are also observed for the other Group 13-15 homologs (R2AlNR’2, R2GaPR’2, etc.).
Borazines
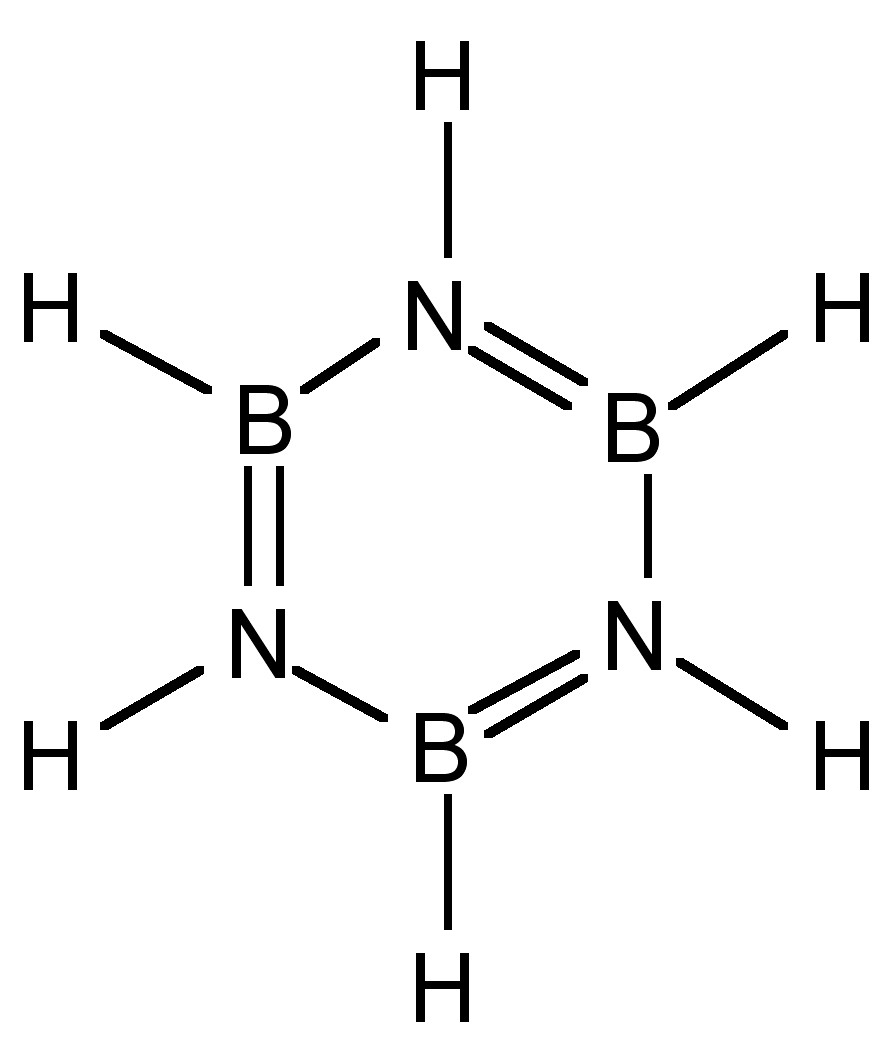
The condensation of boron hydride with ammonia results in the formation of a benzene analog: borazine, (6.7.6). Substituted derivatives are formed by the reaction with primary amines.
\[ \text{BH}_3 \text{ + NH}_3 \rightleftharpoons \text{H}_3\text{B-NH}_3 \xrightarrow[\text{- H}_2]{\Delta} \text{[H}_2\text{B-NH}_2\text{]}_n \xrightarrow[\text{- H}_2]{\Delta} \text{[HBNH]}_6\]
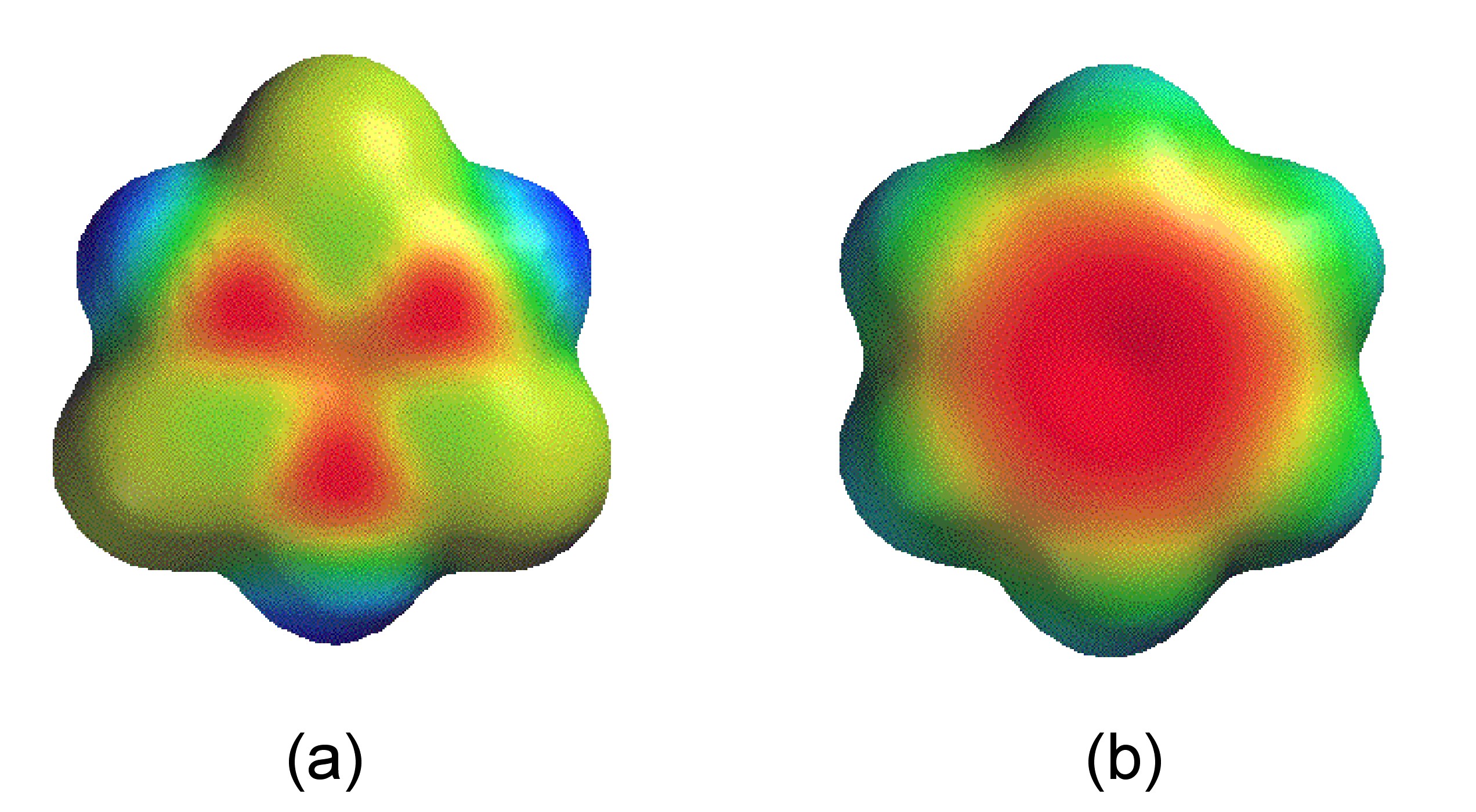
Despite the cyclic structure (Figure \(\PageIndex{3}\)), borazine is not a true analog of benzene. Despite all the B-N bond distances being equal (1.44 Å) consistent with a delocalized structure, the difference in electronegativity of boron and nitrogen (2.04 and 3.04, respectively) results in a polarization of the bonds (i.e., Bδ+-Nδ-) and hence a limit to the delocalization. The molecular orbitals of the π-system in borazine are lumpy in appearance (Figure \(\PageIndex{4}\) a) compared to benzene (Figure \(\PageIndex{4}\) b). This uneven distribution makes borazine prone to addition reactions, making it as a molecule less stable than benzene.
Iminoboranes: analogs of acetylene
Iminoboranes, RB=NR’, are analogs of alkynes, but like aminoboranes are only isolated as monomers with sterically hindered subsistent. In the absence of sufficient steric bulk oligomerization occurs, forming substituted benzene analogs.
Boron nitrides: analogs of elemental carbon
The fusion of borax, Na2[B4O5(OH)4] with ammonium chloride (NH4Cl) results in the formation of hexagonal boron nitride (h-BN). Although h-BN has a planar, layered structure consisting of six-member rings similar to graphite (Figure \(\PageIndex{5}\)), it is a white solid. The difference in color is symptomatic of the more localized bonding in BN than in graphite.
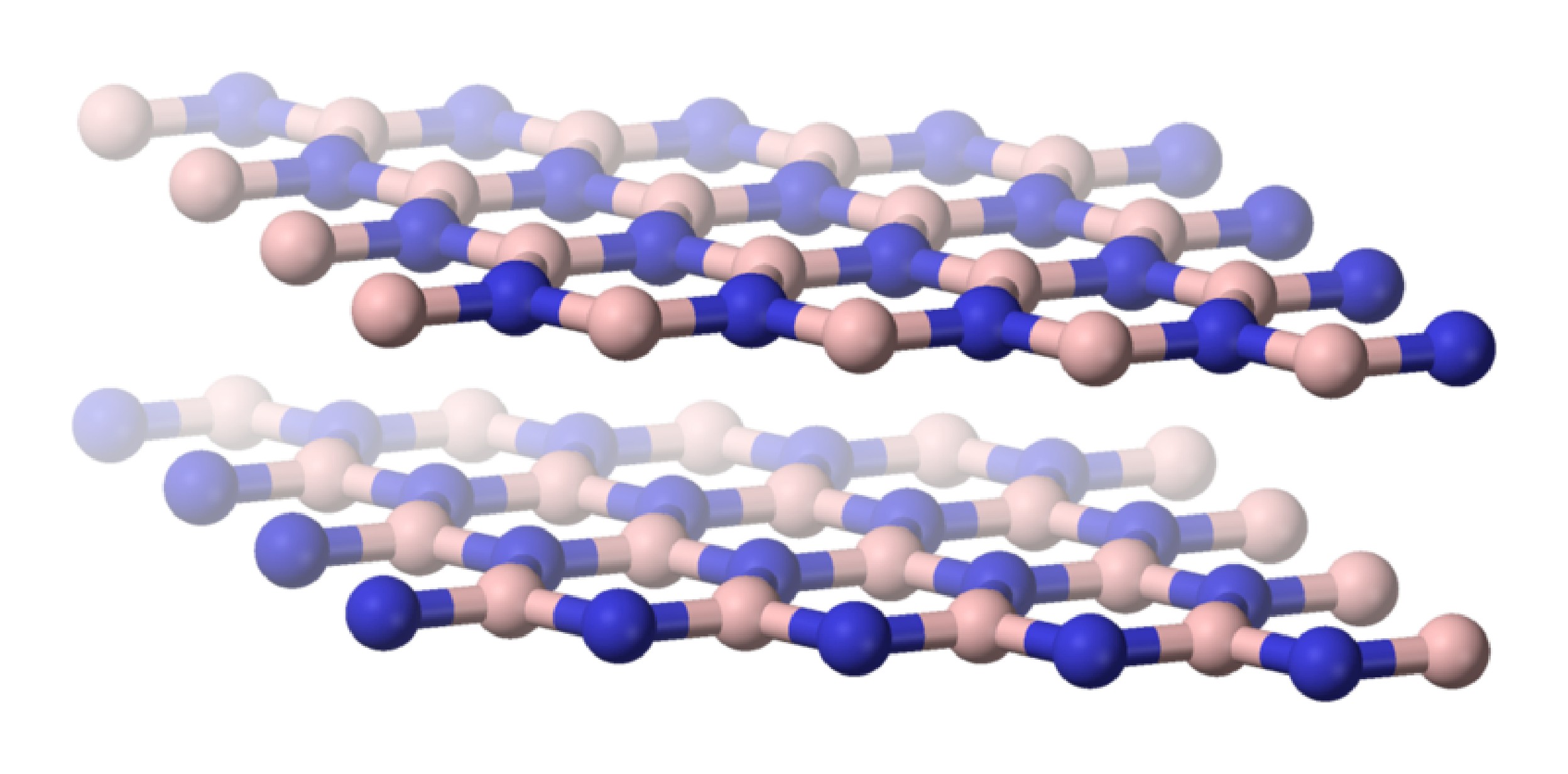
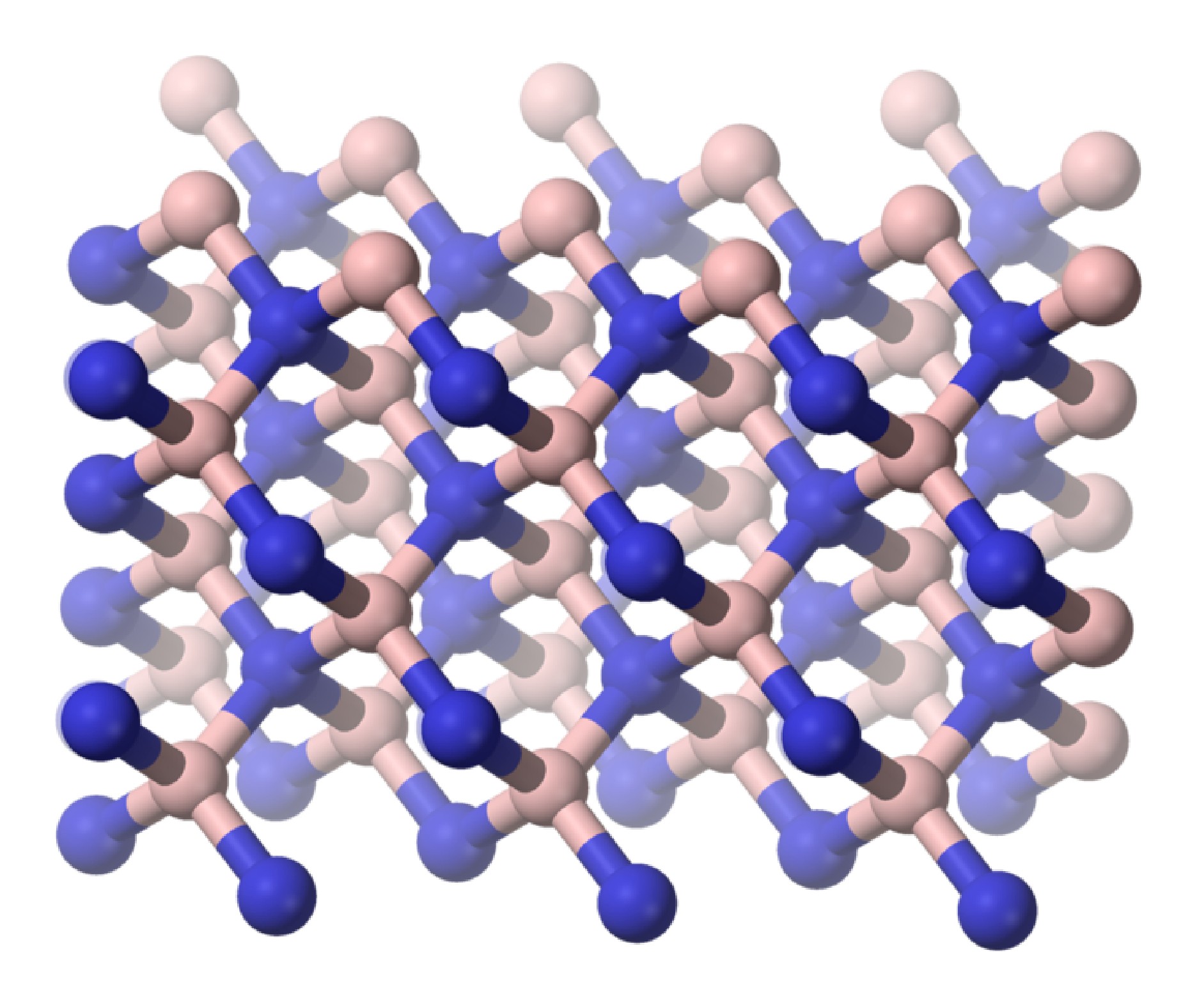
As is found for its carbon analog, hexagonal boron nitride (h-BN or α-BN) is converted at high temperatures (600 – 2000 °C) and pressures (50 – 200 kbar) to a cubic phase (c-BN or β-BN). In a similar manner to diamond, cubic-BN is very hard being actually able to cut diamond, and as a consequence its main use is as an industrial grinding agent. The cubic form has the sphalerite crystal structure (Figure \(\PageIndex{6}\)). Finally, a wurtzite form of boron nitride (w-BN) is known that has similar structure as lonsdaleite, rare hexagonal polymorph of carbon. Table \(\PageIndex{2}\) shows a comparison of the properties of the hexagonal and cubic phases of BN with their carbon analogs.
| Phase | Carbon | Boron nitride |
|---|---|---|
| Cubic | Colorless, hard, mp = 3550 °C. C-C = 1.514 Å | Colorless, hard, B-N = 1.56 Å |
| Hexagonal | Black solid, planar layers, conductor, mp = 3652 – 3697 °C (sublimes), C-C = 1.415 Å | White solid, planar layers, semiconductor (Eg = 5.2 eV), mp = 2973 °C (sublimes), B-N = 1.45 Å |
The partly ionic structure of BN layers in h-BN reduces covalency and electrical conductivity, whereas the interlayer interaction increases resulting in higher hardness of h-BN relative to graphite.
Bibliography
- K. M. Bissett and T. M. Gilbert, Organometallics, 2004, 23, 850.
- P. Paetzold, Adv. Inorg. Chem., 1987, 31, 123.
- L. R. Thorne, R. D. Suenram, and F. J. Lovas, J. Chem. Phys., 1983, 78, 167.

