4.5: Chalcogens and Chalcogenides
- Page ID
- 125395
(a) Simple substances
Sulfur, selenium, and tellurium are called chalcogens. Simple substances and compounds of oxygen and of the elements of this group in the later periods have considerably different properties. As a result of having much smaller electronegativities than oxygen, they show decreased ionicity and increased bond covalency, resulting in a smaller degree of hydrogen bonding. Because they have available d orbitals, chalcogens have increased flexibility of valence and can easily bond to more than two other atoms. Catenation is the bonding between the same chalcogen atoms, and both simple substances and ions of chalcogens take a variety of structures.
The major isotopes of sulfur are 32S (95.02% abundance), 33S (0.75%), 34S (4.21%), and 36S (0.02%), and there are also six radioactive isotopes. Among these, 33S (I = 3/2) can be used for NMR. Since the isotope ratio of sulfurs from different locations differs, the accuracy of the atomic weight is limited to 32.07+0.01. Because the electronegativity of sulfur (\(\chi\) = 2.58) is much smaller than that of oxygen (\(\chi\) = 3.44) and sulfur is a soft element, the ionicity in the bonds of sulfur compounds is low and hydrogen bonding is not important. Elemental sulfur has many allotropes, such as S2, S3, S6, S7, S8, S9, S10, S11, S12, S18, S20, and S\(\alpha\), reflecting the catenation ability of sulfur atoms.
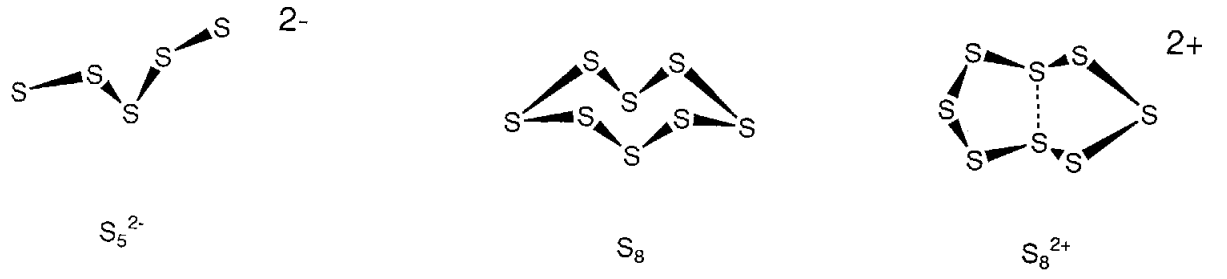
Elemental sulfur is usually a yellow solid with a melting point of 112.8 °C called orthorhombic sulfur (\(\alpha\) sulfur). Phase-transition of this polymorph produces monoclinic sulfur (\(\beta\) sulfur) at 95.5 °C. It was established in 1935 that these are crown-like cyclic molecules (Figure \(\PageIndex{18}\)). Being molecular, they dissolve well in organic solvents, such as CS2. Not only 8-membered rings but also S6-20 rings are known, and the helix polymer of sulfur is an infinitely annular sulfur. Diatomic molecular S2 and triatomic molecular S3 exist in the gaseous phase. When sulfur is heated, it liquifies and becomes a rubber-like macromolecule on cooling. The diversity of structures of catenated sulfur is also seen in the structures of the polysulfur cations or anions resulting from the redox reactions of the catenated species.
Selenium is believed to have six isotopes. 80Se (49.7%) is the most abundant and 77Se, with nuclear spin I = 1/2 is useful in NMR. The accuracy of atomic weight of selenium, 78.96+0.03, is limited to two decimal places because of composition change of its isotopes. Among many allotropes of selenium, so-called red selenium is an Se8 molecule with a crown-like structure and is soluble in CS2. Gray metallic selenium is a polymer with a helical structure. Black selenium, which is a complicated polymer, is also abundant.
Tellurium also has eight stable isotopes and an atomic weight of 127.60+0.03. 130Te (33.8%) and 128Te (31.7%) are the most abundant isotopes, and 125Te and 123Te with I = 1/2 can be used in NMR. There is only one crystalline form of tellurium, which is a spiral chain polymer that shows electric conductivity.
(b) Polyatomic chalcogen cations and anions
Although it has long been recognized that solutions of chalcogen elements in sulfuric acid showed beautiful blue, red, and yellow colors, the polycationic species that give rise to these colors, S42+, S62+, S64+, S82+, S102+, S192+, or those of other chalcogen atoms, have been isolated by the reaction with AsF5, etc. and their structures determined. For example, unlike neutral S8, S82+ takes a cyclic structure that has a weak coupling interaction between two transannular sulfur atoms (Figure \(\PageIndex{18}\)).
On the other hand, alkali metal salts Na2S2, K2S5, and alkaline earth metal salt BaS3, a transition-metal salt [Mo2(S2)6]2-, a complex Cp2W(S4), etc. of polysulfide anions Sx2- (x = 1-6), in which the sulfur atoms are bonded mutually have been synthesized and their structures determined. As is evident from the fact that elemental sulfur itself forms S8 molecules, sulfur, unlike oxygen, tends to catenate. Therefore, formation of polysulfide ions, in which many sulfur atoms are bonded, is feasible, and a series of polysulfanes H2Sx (x = 2-8) has actually been synthesized.
(c) Metal sulfides
Stratified disulfides, MS2, are important in transition metal sulfides. They show two types of structures. One has a metal in a triangular prismatic coordination environment, and the other has a metal in an octahedral coordination environment.
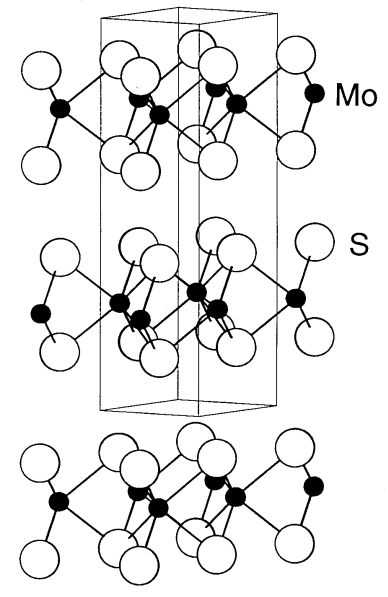
MoS2 is the most stable black compound among the molybdenum sulfides. L. Pauling determined the structure of MoS2 in 1923. The structure is constructed by laminating two sulfur layers between which a molybdenum layer is intercalated (Figure \(\PageIndex{19}\)). Alternatively, two sulfur layers are stacked and a molybdenum layer is inserted between them. Therefore, the coordination environment of each molybdenum is a triangular prism of sulfur atoms. Since there is no bonding interaction between sulfur layers, they can easily slide, resulting in graphite-like lubricity. MoS2 is used as a solid lubricant added to gasoline, and also as a catalyst for hydrogenation reactions.
ZrS2, TaS2, etc. take the CdI2-type structure containing metal atoms in an octahedral coordination environment constructed by sulfur atoms.
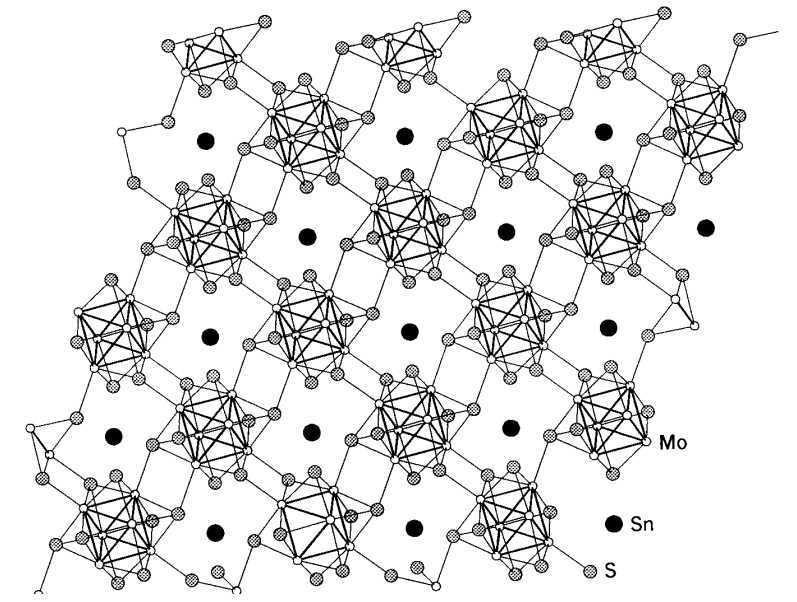
Chevrel phase compounds
There are superconducting compounds called Chevrel phases which are important examples of the chalcogenide compounds of molybdenum. he general formula is described by MxMo6X8 (M = Pb, Sn, and Cu; X = S, Se, and Te), and six molybdenum atoms form a regular octahedral cluster, and eight chalcogenide atoms cap the eight triangular faces of the cluster. The cluster units are connected 3-dimensionally (Figure \(\PageIndex{20}\)). Since the cluster structure of molybdenum atoms is similar to that of molybdenum dichloride, MoCl2, (= (Mo6Cl8)Cl2Cl4/2), the structural chemistry of these compounds has attracted as much attention as their physical properties.