9.7: Acid-Base
- Last updated
- Save as PDF
- Page ID
- 392042
Learning Objectives
- To know the characteristic properties of acids and bases.
Acid–base reactions are essential in both biochemistry and industrial chemistry. Moreover, many of the substances we encounter in our homes, the supermarket, and the pharmacy are acids or bases. For example, aspirin is an acid (acetylsalicylic acid), and antacids are bases. In fact, every amateur chef who has prepared mayonnaise or squeezed a wedge of lemon to marinate a piece of fish has carried out an acid–base reaction. Before we discuss the characteristics of such reactions, let’s first describe some of the properties of acids and bases.
Definitions of Acids and Bases
We can define acids as substances that dissolve in water to produce H+ ions, whereas bases are defined as substances that dissolve in water to produce OH− ions. In fact, this is only one possible set of definitions. Although the general properties of acids and bases have been known for more than a thousand years, the definitions of acid and base have changed dramatically as scientists have learned more about them. In ancient times, an acid was any substance that had a sour taste (e.g., vinegar or lemon juice), caused consistent color changes in dyes derived from plants (e.g., turning blue litmus paper red), reacted with certain metals to produce hydrogen gas and a solution of a salt containing a metal cation, and dissolved carbonate salts such as limestone (CaCO3) with the evolution of carbon dioxide. In contrast, a base was any substance that had a bitter taste, felt slippery to the touch, and caused color changes in plant dyes that differed diametrically from the changes caused by acids (e.g., turning red litmus paper blue). Although these definitions were useful, they were entirely descriptive.
The Arrhenius Definition of Acids and Bases
The first person to define acids and bases in detail was the Swedish chemist Svante Arrhenius (1859–1927; Nobel Prize in Chemistry, 1903). According to the Arrhenius definition, an acid is a substance like hydrochloric acid that dissolves in water to produce H+ ions (protons; Equation \(\ref{4.3.1}\)), and a base is a substance like sodium hydroxide that dissolves in water to produce hydroxide (OH−) ions (Equation \(\ref{4.3.2}\)):
\[ \underset{an\: Arrhenius\: acid}{HCl_{(g)}} \xrightarrow {H_2 O_{(l)}} H^+_{(aq)} + Cl^-_{(aq)} \label{4.3.1} \]
\[ \underset{an\: Arrhenius\: base}{NaOH_{(s)}} \xrightarrow {H_2O_{(l)}} Na^+_{(aq)} + OH^-_{(aq)} \label{4.3.2} \]
According to Arrhenius, the characteristic properties of acids and bases are due exclusively to the presence of H+ and OH− ions, respectively, in solution. Although Arrhenius’s ideas were widely accepted, his definition of acids and bases had two major limitations:
- First, because acids and bases were defined in terms of ions obtained from water, the Arrhenius concept applied only to substances in aqueous solution.
- Second, and more important, the Arrhenius definition predicted that only substances that dissolve in water to produce \(H^+\) and \(OH^−\) ions should exhibit the properties of acids and bases, respectively. For example, according to the Arrhenius definition, the reaction of ammonia (a base) with gaseous HCl (an acid) to give ammonium chloride (Equation \(\ref{4.3.3}\)) is not an acid–base reaction because it does not involve \(H^+\) and \(OH^−\):
\[NH_{3\;(g)} + HCl_{(g)} \rightarrow NH_4Cl_{(s)} \label{4.3.3} \]
The Brønsted–Lowry Definition of Acids and Bases
Because of the limitations of the Arrhenius definition, a more general definition of acids and bases was needed. One was proposed independently in 1923 by the Danish chemist J. N. Brønsted (1879–1947) and the British chemist T. M. Lowry (1874–1936), who defined acid–base reactions in terms of the transfer of a proton (H+ ion) from one substance to another.
According to Brønsted and Lowry, an acid (A substance with at least one hydrogen atom that can dissociate to form an anion and an \(H^+\) ion (a proton) in aqueous solution, thereby forming an acidic solution) is any substance that can donate a proton, and a base (a substance that produces one or more hydroxide ions (\(OH^-\) and a cation when dissolved in aqueous solution, thereby forming a basic solution) is any substance that can accept a proton. The Brønsted–Lowry definition of an acid is essentially the same as the Arrhenius definition, except that it is not restricted to aqueous solutions. The Brønsted–Lowry definition of a base, however, is far more general because the hydroxide ion is just one of many substances that can accept a proton. Ammonia, for example, reacts with a proton to form \(NH_4^+\), so in Equation \(\ref{4.3.3}\), \(NH_3\) is a Brønsted–Lowry base and \(HCl\) is a Brønsted–Lowry acid. Because of its more general nature, the Brønsted–Lowry definition is used throughout this text unless otherwise specified.
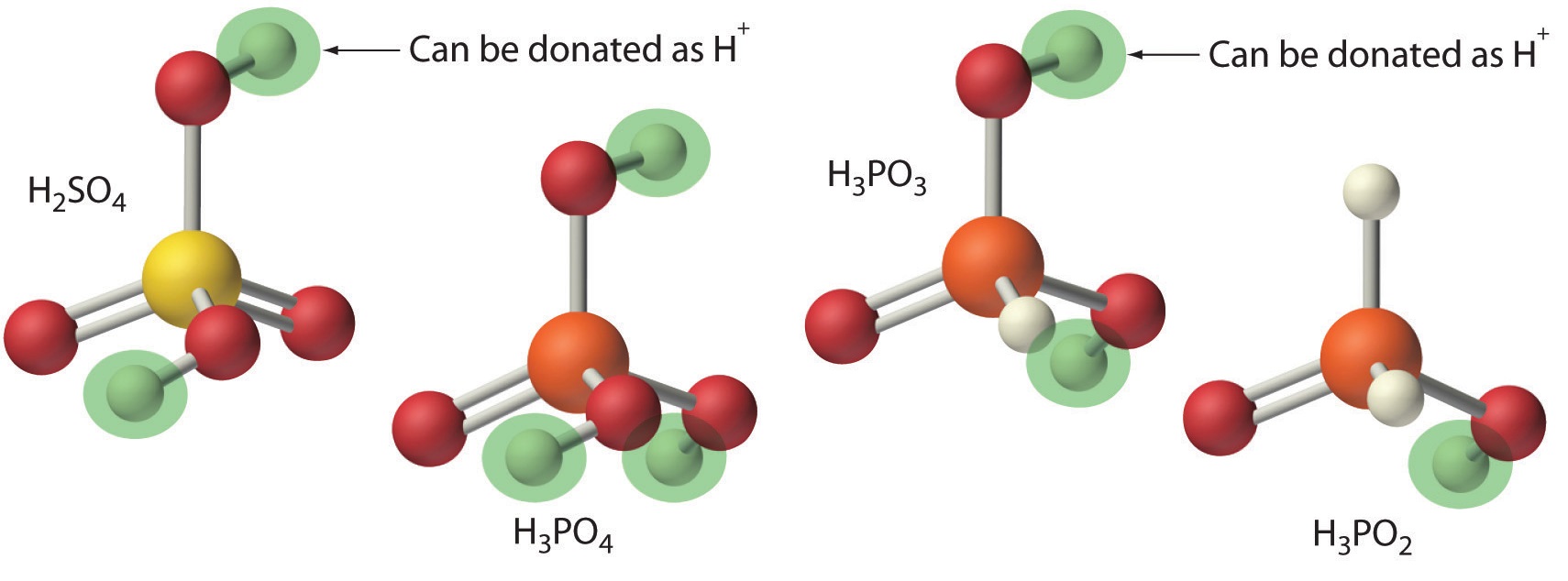
Polyprotic Acids
Acids differ in the number of protons they can donate. For example, monoprotic acids (a compound that is capable of donating one proton per molecule) are compounds that are capable of donating a single proton per molecule. Monoprotic acids include HF, HCl, HBr, HI, HNO3, and HNO2. All carboxylic acids that contain a single −CO2H group, such as acetic acid (CH3CO2H), are monoprotic acids, dissociating to form RCO2− and H+. A compound that can donate more than one proton per molecule is known as a polyprotic acid. For example, H2SO4 can donate two H+ ions in separate steps, so it is a diprotic acid (a compound that can donate two protons per molecule in separate steps) and H3PO4, which is capable of donating three protons in successive steps, is a triprotic acid (a compound that can donate three protons per molecule in separate steps), (Equation \(\ref{4.3.4}\), Equation \(\ref{4.3.5}\), and Equation \(\ref{4.3.6}\)):
\[ H_3 PO_4 (l) \overset{H_2 O(l)}{\rightleftharpoons} H ^+ ( a q ) + H_2 PO_4 ^- (aq) \label{4.3.4} \]
\[ H_2 PO_4 ^- (aq) \rightleftharpoons H ^+ (aq) + HPO_4^{2-} (aq) \label{4.3.5} \]
\[ HPO_4^{2-} (aq) \rightleftharpoons H^+ (aq) + PO_4^{3-} (aq) \label{4.3.6} \]
In chemical equations such as these, a double arrow is used to indicate that both the forward and reverse reactions occur simultaneously, so the forward reaction does not go to completion. Instead, the solution contains significant amounts of both reactants and products. Over time, the reaction reaches a state in which the concentration of each species in solution remains constant. The reaction is then said to be in equilibrium (the point at which the rates of the forward and reverse reactions become the same, so that the net composition of the system no longer changes with time).
Strengths of Acids and Bases
We will not discuss the strengths of acids and bases quantitatively until next semester. Qualitatively, however, we can state that strong acids react essentially completely with water to give \(H^+\) and the corresponding anion. Similarly, strong bases dissociate essentially completely in water to give \(OH^−\) and the corresponding cation. Strong acids and strong bases are both strong electrolytes. In contrast, only a fraction of the molecules of weak acids and weak bases react with water to produce ions, so weak acids and weak bases are also weak electrolytes. Typically less than 5% of a weak electrolyte dissociates into ions in solution, whereas more than 95% is present in undissociated form.
In practice, only a few strong acids are commonly encountered: HCl, HBr, HI, HNO3, HClO4, and H2SO4 (H3PO4 is only moderately strong). The most common strong bases are ionic compounds that contain the hydroxide ion as the anion; three examples are NaOH, KOH, and Ca(OH)2. Common weak acids include HCN, H2S, HF, oxoacids such as HNO2 and HClO, and carboxylic acids such as acetic acid. The ionization reaction of acetic acid is as follows:
\[ CH_3 CO_2 H(l) \overset{H_2 O(l)}{\rightleftharpoons} H^+ (aq) + CH_3 CO_2^- (aq) \label{4.3.7} \]
Although acetic acid is very soluble in water, almost all of the acetic acid in solution exists in the form of neutral molecules (less than 1% dissociates). Sulfuric acid is unusual in that it is a strong acid when it donates its first proton (Equation \(\ref{4.3.8}\)) but a weak acid when it donates its second proton (Equation \(\ref{4.3.9}\)) as indicated by the single and double arrows, respectively:
\[ \underset{strong\: acid}{H_2 SO_4 (l)} \xrightarrow {H_2 O(l)} H ^+ (aq) + HSO_4 ^- (aq) \label{4.3.8} \]
\[ \underset{weak\: acid}{HSO_4^- (aq)} \rightleftharpoons H^+ (aq) + SO_4^{2-} (aq) \label{4.3.9} \]
Consequently, an aqueous solution of sulfuric acid contains \(H^+_{(aq)}\) ions and a mixture of \(HSO^-_{4\;(aq)}\) and \(SO^{2−}_{4\;(aq)}\) ions, but no \(H_2SO_4\) molecules. All other polyprotic acids, such as H3PO4, are weak acids.
The most common weak base is ammonia, which reacts with water to form small amounts of hydroxide ion:
\[ NH_3 (g) + H_2 O(l) \rightleftharpoons NH_4^+ (aq) + OH^- (aq) \label{4.3.10} \]
Most of the ammonia (>99%) is present in the form of NH3(g). Amines, which are organic analogues of ammonia, are also weak bases, as are ionic compounds that contain anions derived from weak acids (such as S2−).
There is no correlation between the solubility of a substance and whether it is a strong electrolyte, a weak electrolyte, or a nonelectrolyte.
Definition of Strong/Weak Acids & Bases: Definition of Strong/Weak Acids & Bases, YouTube (opens in new window) [Definition of Strong] [Definition of Strong] [youtu.be] (opens in new window)
Table \(\PageIndex{1}\) lists some common strong acids and bases. Acids other than the six common strong acids are almost invariably weak acids. The only common strong bases are the hydroxides of the alkali metals and the heavier alkaline earths (Ca, Sr, and Ba); any other bases you encounter are most likely weak. Remember that there is no correlation between solubility and whether a substance is a strong or a weak electrolyte! Many weak acids and bases are extremely soluble in water.
| Strong Acids | Strong Bases | ||
|---|---|---|---|
| Hydrogen Halides | Oxoacids | Group 1 Hydroxides | Hydroxides of Heavy Group 2 Elements |
| HCl | HNO3 | LiOH | Ca(OH)2 |
| HBr | H2SO4 | NaOH | Sr(OH)2 |
| HI | HClO4 | KOH | Ba(OH)2 |
| RbOH | |||
| CsOH | |||
Example \(\PageIndex{1}\): Acid Strength
Classify each compound as a strong acid, a weak acid, a strong base, a weak base, or none of these.
- CH3CH2CO2H
- CH3OH
- Sr(OH)2
- CH3CH2NH2
- HBrO4
Given: compound
Asked for: acid or base strength
Strategy:
A Determine whether the compound is organic or inorganic.
B If inorganic, determine whether the compound is acidic or basic by the presence of dissociable H+ or OH− ions, respectively. If organic, identify the compound as a weak base or a weak acid by the presence of an amine or a carboxylic acid group, respectively. Recall that all polyprotic acids except H2SO4 are weak acids.
Solution:
- A This compound is propionic acid, which is organic. B It contains a carboxylic acid group analogous to that in acetic acid, so it must be a weak acid.
- A CH3OH is methanol, an organic compound that contains the −OH group. B As a covalent compound, it does not dissociate to form the OH− ion. Because it does not contain a carboxylic acid (−CO2H) group, methanol also cannot dissociate to form H+(aq) ions. Thus we predict that in aqueous solution methanol is neither an acid nor a base.
- A Sr(OH)2 is an inorganic compound that contains one Sr2+ and two OH− ions per formula unit. B We therefore expect it to be a strong base, similar to Ca(OH)2.
- A CH3CH2NH2 is an amine (ethylamine), an organic compound in which one hydrogen of ammonia has been replaced by an R group. B Consequently, we expect it to behave similarly to ammonia (Equation \(\ref{4.3.7}\)), reacting with water to produce small amounts of the OH− ion. Ethylamine is therefore a weak base.
- A HBrO4 is perbromic acid, an inorganic compound. B It is not listed in Table \(\PageIndex{1}\) as one of the common strong acids, but that does not necessarily mean that it is a weak acid. If you examine the periodic table, you can see that Br lies directly below Cl in group 17. We might therefore expect that HBrO4 is chemically similar to HClO4, a strong acid—and, in fact, it is.
Exercise \(\PageIndex{1}\): Acid Strength
Classify each compound as a strong acid, a weak acid, a strong base, a weak base, or none of these.
- Ba(OH)2
- HIO4
- CH3CH2CH2CO2H
- (CH3)2NH
- CH2O
- Answer a
-
strong base
- Answer b
-
strong acid
- Answer c
-
weak acid
- Answer d
-
weak base
- Answer e
-
none of these; formaldehyde is a neutral molecule

