8.7: Bonding in Coordination Complexes
- Page ID
- 41577
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Ligands can be further characterized as monodentate, bidentate, tridentate etc. where the concept of teeth (dent) is introduced. Monodentate ligands bind through only one donor atom. Monodentate means "one-toothed." The halides, phosphines, ammonia and amines seen previously are monodentate ligands. Bidentate ligands bind through two donor sites. Bidentate means "two-toothed." An example of a bidentate ligand is ethylenediamine. It can bind to a metal via two donor atoms at once.

Bidentate binding allows a ligand to bind more tightly. Tridentate ligands, which bind through three donors, can bind even more tightly, and so on. This phenomenon is generally called the "chelate effect." This term comes from the Greek chelos, meaning "crab." A crab does not have any teeth at all, but it does have two claws for tightly holding onto something for a couple of reasons. A very simple analogy is that, if you are holding something with two hands rather than one, you are not as likely to drop it.
Complex metal ions containing more complicated ligands
In the examples previously disccussed, each ligand only forms one bond with the central metal ion to give the complex ion. Such a ligand is said to be unidentate. That means literally that it only has one tooth! It only has one pair of electrons that it can use to bond to the metal - any other lone pairs are pointing in the wrong direction. Some ligands, however, have rather more teeth! These are known generally as multidentate or polydentate ligands, but can be broken down into a number of different types.
Bidentate ligands
Bidentate ligands have two lone pairs, both of which can bond to the central metal ion. The two commonly used examples are 1,2-diaminoethane (old name: ethylenediamine - often given the abbreviation "en"), and the ethanedioate ion (old name: oxalate).
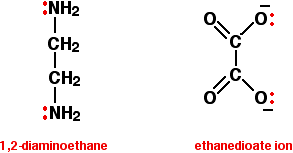
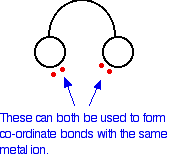
In the ethanedioate ion, there are lots more lone pairs than the two shown, but these are the only ones we are interested in. You can think of these bidentate ligands rather as if they were a pair of headphones, carrying lone pairs on each of the "ear pieces". These will then fit snuggly around a metal ion.
You might find this abbreviated to \([Ni(en)_3]^{2+}\). The structure of the ion looks like this:
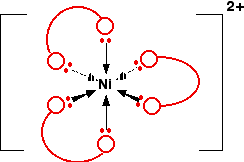
In this case, the "ear pieces" are the nitrogen atoms of the NH2 groups - and the "bit that goes over your head " is the \(-CH_2CH_2-\) group. If you were going to draw this in an exam, you would obviously want to draw it properly - but for learning purposes, drawing all the atoms makes the diagram look unduly complicated!
Notice that the arrangement of the bonds around the central metal ion is exactly the same as it was with the ions with 6 water molecules attached. The only difference is that this time each ligand uses up two of the positions - at right angles to each other.
Because the nickel is forming 6 co-ordinate bonds, the coordination number of this ion is 6, despite the fact that it is only joined to 3 ligands. Coordination number counts the number of bonds, not the number of ligands.
This is the complex ion formed by attaching 3 ethanedioate (oxalate) ions to a chromium(III) ion. The shape is exactly the same as the previous nickel complex. The only real difference is the number of charges. The original chromium ion carried 3+ charges, and each ethanedioate ion carried -2, i.e.,
\[ (+3) + (3 \times -2) = -3. \nonumber \]
The structure of the ion looks like this:
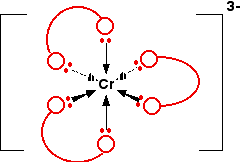
Again, if you drew this in an exam, you would want to show all the atoms properly. If you need to be able to do this, practice drawing it so that it looks clear and tidy! Refer back to the diagram of the ethanedioate ion further up the page to help you.
A Quadridentate Ligand
A quadridentate ligand has four lone pairs, all of which can bond to the central metal ion. An example of this occurs in haemoglobin (American: hemoglobin). The functional part of this is an iron(II) ion surrounded by a complicated molecule called heme. This is a sort of hollow ring of carbon and hydrogen atoms, at the center of which are 4 nitrogen atoms with lone pairs on them. Heme is one of a group of similar compounds called porphyrins. They all have the same sort of ring system, but with different groups attached to the outside of the ring. You aren't going to need to know the exact structure of the haem at this level.
We could simplify the heme with the trapped iron ion as:
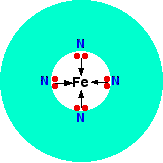
Each of the lone pairs on the nitrogen can form a co-ordinate bond with the iron(II) ion - holding it at the center of the complicated ring of atoms. The iron forms 4 co-ordinate bonds with the heme, but still has space to form two more - one above and one below the plane of the ring. The protein globin attaches to one of these positions using a lone pair on one of the nitrogens in one of its amino acids. The interesting bit is the other position.
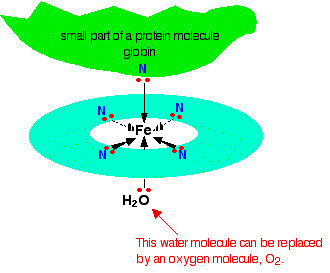
The water molecule which is bonded to the bottom position in the diagram is easily replaced by an oxygen molecule (again via a lone pair on one of the oxygens in \(O_2\)) - and this is how oxygen gets carried around the blood by the haemoglobin. When the oxygen gets to where it is needed, it breaks away from the haemoglobin which returns to the lungs to get some more.
You probably know that carbon monoxide is poisonous because it reacts with hemeoglobin. It bonds to the same site that would otherwise be used by the oxygen - but it forms a very stable complex. The carbon monoxide doesn't break away again, and that makes that hemeoglobin molecule useless for any further oxygen transfer.
A Hexadentate Ligand
A hexadentate ligand has 6 lone pairs of electrons - all of which can form co-ordinate bonds with the same metal ion. The best example is EDTA. The diagram shows the structure of the ion with the important atoms and lone pairs picked out.
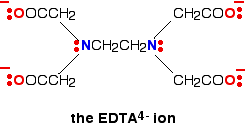
The EDTA ion entirely wraps up a metal ion using all 6 of the positions that we have seen before. The co-ordination number is again 6 because of the 6 co-ordinate bonds being formed by the central metal ion. The diagram below shows this happening with a copper(II) ion. Here is a simplified version. Make sure that you can see how this relates to the full structure above.
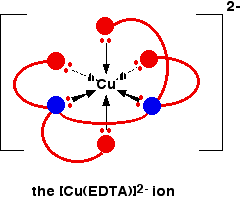
The overall charge, of course, comes from the 2+ on the original copper(II) ion and the 4- on the \(EDTA^{4-}\) ion.
Contributors and Attributions
Jim Clark (Chemguide.co.uk)

