2.15: Determining Formulas from Mass Data
- Page ID
- 13567
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Stoichiometry is a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. In order to use stoichiometry to run calculations about chemical reactions, it is important to first understand the relationships that exist between products and reactants and why they exist, which require understanding how to balance reactions.
Balancing
In chemistry, chemical reactions are frequently written as an equation, using chemical symbols. The reactants are displayed on the left side of the equation and the products are shown on the right, with the separation of either a single or double arrow that signifies the direction of the reaction. The significance of single and double arrow is important when discussing solubility constants, but we will not go into detail about it in this module. To balance an equation, it is necessary that there are the same number of atoms on the left side of the equation as the right. One can do this by raising the coefficients.
Reactants to Products
A chemical equation is like a recipe for a reaction so it displays all the ingredients or terms of a chemical reaction. It includes the elements, molecules, or ions in the reactants and in the products as well as their states, and the proportion for how much of each particle reacts or is formed relative to one another, through the stoichiometric coefficient. The following equation demonstrates the typical format of a chemical equation:
\[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber \]
In the above equation, the elements present in the reaction are represented by their chemical symbols. Based on the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction, every chemical reaction has the same elements in its reactants and products, though the elements they are paired up with often change in a reaction. In this reaction, sodium (\(Na\)), hydrogen (\(H\)), and chloride (\(Cl\)) are the elements present in both reactants, so based on the law of conservation of mass, they are also present on the product side of the equations. Displaying each element is important when using the chemical equation to convert between elements.
Stoichiometric Coefficients
In a balanced reaction, both sides of the equation have the same number of elements. The stoichiometric coefficient is the number written in front of atoms, ion and molecules in a chemical reaction to balance the number of each element on both the reactant and product sides of the equation. Though the stoichiometric coefficients can be fractions, whole numbers are frequently used and often preferred. This stoichiometric coefficients are useful since they establish the mole ratio between reactants and products. In the balanced equation:
\[\ce{2 Na(s) + 2HCl(aq) \rightarrow 2NaCl(aq) + H2(g)} \nonumber \]
we can determine that 2 moles of \(HCl\) will react with 2 moles of \(Na_{(s)}\) to form 2 moles of \(NaCl_{(aq)}\) and 1 mole of \(H_{2(g)}\). If we know how many moles of \(Na\) reacted, we can use the ratio of 2 moles of \(NaCl\) to 2 moles of Na to determine how many moles of \(NaCl\) were produced or we can use the ratio of 1 mole of \(H_2\) to 2 moles of \(Na\) to convert to \(NaCl\). This is known as the coefficient factor. The balanced equation makes it possible to convert information about the change in one reactant or product to quantitative data about another reactant or product. Understanding this is essential to solving stoichiometric problems.
Lead (IV) hydroxide and sulfuric acid react as shown below. Balance the reaction.
\[\ce{Pb(OH)4 + H2SO4 \rightarrow Pb(SO4)2 +H2O} \nonumber \]
Solution
Start by counting the number of atoms of each element.
UNBALANCED
|
Element |
Reactant (# of atoms) |
Product (# of atoms) |
|---|---|---|
|
Pb |
1 |
1 |
|
O |
8 |
9 |
|
H |
6 |
2 |
|
S |
1 |
2 |
The reaction is not balanced; the reaction has 16 reactant atoms and only 14 product atoms and does not obey the conservation of mass principle. Stoichiometric coefficients must be added to make the equation balanced. In this example, there are only one sulfur atom present on the reactant side, so a coefficient of 2 should be added in front of \(H_2SO_4\) to have an equal number of sulfur on both sides of the equation. Since there are 12 oxygen on the reactant side and only 9 on the product side, a 4 coefficient should be added in front of \(H_2O\) where there is a deficiency of oxygen. Count the number of elements now present on either side of the equation. Since the numbers are the same, the equation is now balanced.
\[\ce{ Pb(OH)4 + 2 H2SO4 \rightarrow Pb(SO4)2 + 4H2O} \nonumber \]
BALANCED
|
Element |
Reactant (# of atoms) |
Product (# of atoms) |
|---|---|---|
|
Pb |
1 |
1 |
|
O |
12 |
12 |
|
H |
8 |
8 |
|
S |
2 |
2 |
Balancing reactions involves finding least common multiples between numbers of elements present on both sides of the equation. In general, when applying coefficients, add coefficients to the molecules or unpaired elements last.
A balanced equation ultimately has to satisfy two conditions.
- The numbers of each element on the left and right side of the equation must be equal.
- The charge on both sides of the equation must be equal. It is especially important to pay attention to charge when balancing redox reactions.
Stoichiometry and Balanced Equations
In stoichiometry, balanced equations make it possible to compare different elements through the stoichiometric factor discussed earlier. This is the mole ratio between two factors in a chemical reaction found through the ratio of stoichiometric coefficients. Here is a real world example to show how stoichiometric factors are useful.
There are 12 party invitations and 20 stamps. Each party invitation needs 2 stamps to be sent. How many party invitations can be sent?
Solution
The equation for this can be written as
\[\ce{I + 2S \rightarrow IS2}\nonumber \]
where
- \(I\) represents invitations,
- \(S\) represents stamps, and
- \(IS_2\) represents the sent party invitations consisting of one invitation and two stamps.
Based on this, we have the ratio of 2 stamps for 1 sent invite, based on the balanced equation.
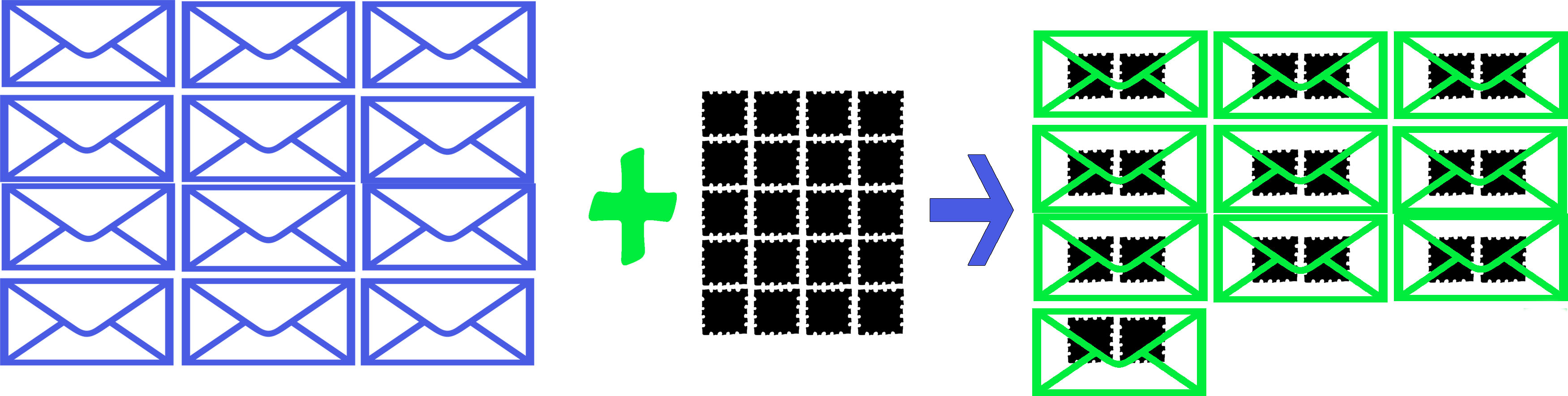
Invitations Stamps Party Invitations Sent
In this example are all the reactants (stamps and invitations) used up? No, and this is normally the case with chemical reactions. There is often excess of one of the reactants. The limiting reagent, the one that runs out first, prevents the reaction from continuing and determines the maximum amount of product that can be formed.
What is the limiting reagent in this example?
Solution
Stamps, because there was only enough to send out invitations, whereas there were enough invitations for 12 complete party invitations. Aside from just looking at the problem, the problem can be solved using stoichiometric factors.
12 I x (1IS2/1I) = 12 IS2 possible
20 S x (1IS2/2S) = 10 IS2 possible
When there is no limiting reagent because the ratio of all the reactants caused them to run out at the same time, it is known as stoichiometric proportions.
Types of Reactions
There are 6 basic types of reactions.
- Combustion: Combustion is the formation of CO2 and H2O from the reaction of a chemical and O2
- Combination (synthesis): Combination is the addition of 2 or more simple reactants to form a complex product.
- Decomposition: Decomposition is when complex reactants are broken down into simpler products.
- Single Displacement: Single displacement is when an element from on reactant switches with an element of the other to form two new reactants.
- Double Displacement: Double displacement is when two elements from on reactants switched with two elements of the other to form two new reactants.
- Acid-Base: Acid- base reactions are when two reactants form salts and water.
Molar Mass
Before applying stoichiometric factors to chemical equations, you need to understand molar mass. Molar mass is a useful chemical ratio between mass and moles. The atomic mass of each individual element as listed in the periodic table established this relationship for atoms or ions. For compounds or molecules, you have to take the sum of the atomic mass times the number of each atom in order to determine the molar mass
What is the molar mass of H2O?
Solution
\[\text{Molar mass} = 2 \times (1.00794\; g/mol) + 1 \times (15.9994\; g/mol) = 18.01528\; g/mol \nonumber \]
Using molar mass and coefficient factors, it is possible to convert mass of reactants to mass of products or vice versa.
Propane (\(\ce{C_3H_8}\)) burns in this reaction:
\[\ce{C_3H_8 + 5O_2 \rightarrow 4H_2O + 3CO_2} \nonumber \]
If 200 g of propane is burned, how many g of \(H_2O\) is produced?
Solution
Steps to getting this answer: Since you cannot calculate from grams of reactant to grams of products you must convert from grams of \(C_3H_8\) to moles of \(C_3H_8\) then from moles of \(C_3H_8\) to moles of \(H_2O\). Then convert from moles of \(H_2O\) to grams of \(H_2O\).
- Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\).
- Step 2: Since there is a ratio of 4:1 \(H_2O\) to \(C_3H_8\), for every 4.54 mol \(C_3H_8\) there are 18.18 mol \(H_2O\).
- Step 3: Convert 18.18 mol \(H_2O\) to g \(H_2O\). 18.18 mol \(H_2O\) is equal to 327.27 g \(H_2O\).
Variation in Stoichiometric Equations
Almost every quantitative relationship can be converted into a ratio that can be useful in data analysis.
Density
Density (\(\rho\)) is calculated as mass/volume. This ratio can be useful in determining the volume of a solution, given the mass or useful in finding the mass given the volume. In the latter case, the inverse relationship would be used.
Volume x (Mass/Volume) = Mass
Mass x (Volume/Mass) = Volume
Percent Mass
Percents establish a relationship as well. A percent mass states how many grams of a mixture are of a certain element or molecule. The percent X% states that of every 100 grams of a mixture, X grams are of the stated element or compound. This is useful in determining mass of a desired substance in a molecule.
A substance is 5% carbon by mass. If the total mass of the substance is 10 grams, what is the mass of carbon in the sample? How many moles of carbon are there?
Solution
10 g sample x (5 g carbon/100 g sample) = 0.5 g carbon
0.5g carbon x (1 mol carbon/12.011g carbon) = 0.0416 mol carbon
Molarity
Molarity (moles/L) establishes a relationship between moles and liters. Given volume and molarity, it is possible to calculate mole or use moles and molarity to calculate volume. This is useful in chemical equations and dilutions.
How much 5 M stock solution is needed to prepare 100 mL of 2 M solution?
Solution
100 mL of dilute solution (1 L/1000 mL)(2 mol/1L solution)(1 L stock solution/5 mol solution)(1000 ml stock solution/1L stock solution) = 40 mL stock solution.
These ratios of molarity, density, and mass percent are useful in complex examples ahead.
Determining Empirical Formulas
An empirical formula can be determined through chemical stoichiometry by determining which elements are present in the molecule and in what ratio. The ratio of elements is determined by comparing the number of moles of each element present.
1.000 gram of an organic molecule burns completely in the presence of excess oxygen. It yields 0.0333 mol of CO2 and 0.599 g of H2O. What is the empirical formula of the organic molecule?
Solution
This is a combustion reaction. The problem requires that you know that organic molecules consist of some combination of carbon, hydrogen, and oxygen elements. With that in mind, write the chemical equation out, replacing unknown numbers with variables. Do not worry about coefficients here.
\[ \ce{C_xH_yO_z(g) + O_2(g) \rightarrow CO_2(g) + H_2O(g)} \nonumber \]
Since all the moles of C and H in CO2 and H2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample.
0.0333mol CO2 (1mol C/ 1mol CO2) = 0.0333mol C in unknown
0.599g H2O (1mol H2O/ 18.01528g H2O)(2mol H/ 1mol H2O) = 0.0665 mol H in unknown
Calculate the final moles of oxygen by taking the sum of the moles of oxygen in CO2 and H2O. This will give you the number of moles from both the unknown organic molecule and the O2 so you must subtract the moles of oxygen transferred from the O2.
Moles of oxygen in CO2:
0.0333mol CO2 (2mol O/1mol CO2) = 0.0666 mol O
Moles of oxygen in H2O:
0.599g H2O (1mol H2O/18.01528 g H2O)(1mol O/1mol H2O) = 0.0332 mol O
Using the Law of Conservation, we know that the mass before a reaction must equal the mass after a reaction. With this we can use the difference of the final mass of products and initial mass of the unknown organic molecule to determine the mass of the O2 reactant.
0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2
1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2
Moles of oxygen in O2
1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O
Moles of oxygen in unknown
(0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O
Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number.
(1/0.0332)(0.0333mol C : 0.0665mol H : 0.0332 mol O) => 1mol C: 2 mol H: 1 mol O
From this ratio, the empirical formula is calculated to be CH2O.
Determining Molecular Formulas
To determine a molecular formula, first determine the empirical formula for the compound as shown in the section above and then determine the molecular mass experimentally. Next, divide the molecular mass by the molar mass of the empirical formula (calculated by finding the sum the total atomic masses of all the elements in the empirical formula). Multiply the subscripts of the molecular formula by this answer to get the molecular formula.
In the example above, it was determined that the unknown molecule had an empirical formula of CH2O.
1. Find the molar mass of the empircal formula CH2O.
12.011g C + (1.008 g H) * (2 H) + 15.999g O = 30.026 g/mol CH2O
2. Determine the molecular mass experimentally. For our compound, it is 120.056 g/mol.
3. Divide the experimentally determined molecular mass by the mass of the empirical formula.
(120.056 g/mol) / (30.026 g/mol) = 3.9984
4. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. If the answer is not close to a whole number, there was either an error in the calculation of the empirical formula or a large error in the determination of the molecular mass.
5. Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula.
CH2O * 4 = ?
C: 1 * 4 = 4
H: 2 * 4 = 8
O 1 * 4 = 4
CH2O * 4 = C4H8O4
6. Check your result by calculating the molar mass of the molecular formula and comparing it to the experimentally determined mass.
molar mass of C4H8O4= 120.104 g/mol
experimentally determined mass = 120.056 g/mol
% error = | theoretical - experimental | / theoretical * 100%
% error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%
% error = 0.040 %
An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. The alloy's density is 3.15 g/L. One liter of alloy completely fills a mold of volume 1000 cm3. He accidentally breaks off a 1.203 cm3 piece of the homogenous mixture and sweeps it outside where it reacts with acid rain over years. Assuming the acid reacts with all the iron(II) and not with the copper, how many grams of H2(g) are released into the atmosphere because of the amateur's carelessness? (Note that the situation is fiction.)
Solution
Step 1: Write a balanced equation after determining the products and reactants. In this situation, since we assume copper does not react, the reactants are only H+(aq) and Fe(s). The given product is H2(g) and based on knowledge of redox reactions, the other product must be Fe2+(aq).
\[\ce{Fe(s) + 2H^{+}(aq) \rightarrow H2(g) + Fe^{2+}(aq)} \nonumber \]
Step 2: Write down all the given information
Alloy density = (3.15g alloy/ 1L alloy)
x grams of alloy = 45% copper = (45g Cu(s)/100g alloy)
x grams of alloy = 55% iron(II) = (55g Fe(s)/100g alloy)
1 liter alloy = 1000cm3 alloy
alloy sample = 1.203cm3 alloy
Step 3: Answer the question of what is being asked. The question asks how much H2(g) was produced. You are expected to solve for the amount of product formed.
Step 4: Start with the compound you know the most about and use given ratios to convert it to the desired compound.
Convert the given amount of alloy reactant to solve for the moles of Fe(s) reacted.
1.203cm3 alloy(1liter alloy/1000cm3 alloy)(3.15g alloy/1liter alloy)(55g Fe(s)/100g alloy)(1mol Fe(s)/55.8g Fe(s))=3.74 x 10-5 mol Fe(s)
Make sure all the units cancel out to give you moles of \(\ce{Fe(s)}\). The above conversion involves using multiple stoichiometric relationships from density, percent mass, and molar mass.
The balanced equation must now be used to convert moles of Fe(s) to moles of H2(g). Remember that the balanced equation's coefficients state the stoichiometric factor or mole ratio of reactants and products.
3.74 x 10-5 mol Fe (s) (1mol H2(g)/1mol Fe(s)) = 3.74 x 10-5 mol H2(g)
Step 5: Check units
The question asks for how many grams of H2(g) were released so the moles of H2(g) must still be converted to grams using the molar mass of H2(g). Since there are two H in each H2, its molar mass is twice that of a single H atom.
molar mass = 2(1.00794g/mol) = 2.01588g/mol
3.74 x 10-5 mol H2(g) (2.01588g H2(g)/1mol H2 (g)) = 7.53 x 10-5 g H2(g) released
Problems
Stoichiometry and balanced equations make it possible to use one piece of information to calculate another. There are countless ways stoichiometry can be used in chemistry and everyday life. Try and see if you can use what you learned to solve the following problems.
1) Why are the following equations not considered balanced?
- \(H_2O_{(l)} \rightarrow H_{2(g)} + O_{2(g)}\)
- \(Zn_{(s)} + Au^+_{(aq)} \rightarrow Zn^{2+}_{(aq)} + Ag_{(s)}\)
2) Hydrochloric acid reacts with a solid chunk of aluminum to produce hydrogen gas and aluminum ions. Write the balanced chemical equation for this reaction.
3) Given a 10.1M stock solution, how many mL must be added to water to produce 200 mL of 5M solution?
4) If 0.502g of methane gas react with 0.27g of oxygen to produce carbon dioxide and water, what is the limiting reagent and how many moles of water are produced? The unbalanced equation is provided below.
\[\ce{CH4(g) + O2(g) \rightarrow CO2(g) + H2O(l)} \nonumber \]
5) A 0.777g sample of an organic compound is burned completely. It produces 1.42g CO2 and 0.388g H2O. Knowing that all the carbon and hydrogen atoms in CO2 and H2O came from the 0.777g sample, what is the empirical formula of the organic compound?
Weblinks for further reference
- 1. Refer to http://chemistry.about.com/cs/stoich.../aa042903a.htm as an outside resource on how to balance chemical reactions.
- 2. Refer to http://www.learnchem.net/tutorials/stoich.shtml as an outside resource on stoichiometry.
References
- T. E. Brown, H.E LeMay, B. Bursten, C. Murphy. Chemistry: The Central Science. Prentice Hall, January 8, 2008.
- J. C. Kotz P.M. Treichel, J. Townsend. Chemistry and Chemical Reactivity. Brooks Cole, February 7, 2008.
- Petrucci, harwood, Herring, Madura. General Chemistry Principles & Modern Applications. Prentice Hall. New Jersey, 2007.
Contributors and Attributions
- Joseph Nijmeh (UCD), Mark Tye (DVC)

