Chemical nomenclature is the names we use for chemicals. For instance, H2O is called "water", and CH4 (the gas you burn in a stove) is called "methane." You should learn the chemical nomenclature here on this page now, so that you will be able to understand when it is used.
The Basics
Here is some important info about how we write chemicals.
- Elements have symbols of one or two letters. The first is a capital letter (ABC). If there is a second letter, it is a lower-case letter (abc). For instance, "m" is one unit, and "M" is a different unit. "K" is the symbol for one type of constant, and "k" is the symbol for a different type. You need to remember that capital letter symbols are usually different from lower-case symbols. For instance, Co is cobalt, a metal element next to iron, and CO is carbon monoxide, a poisonous gas made of one carbon atom and one oxygen atom.

- We write the charge of a chemical using a superscript, which looks like this: H+or H–. If we write just H, that means an H atom, which is one proton and one electron. H+ means 1 H atom – 1 electron, so it means just one proton, also called hydrogen ion. If we write H– this means one hydrogen atom + one electron, so a proton and 2 electrons, also called hydride ion. If there's an number in the superscript, that says how many electrons are added or removed. For instance, Ca2+ is a calcium atom – 2 electrons, or calcium ion. S2– is sulfide, or sulfur + 2 electrons.

- We indicate the number of atoms of a particular type using a subscript, like this: CO2. This means one carbon atom and 2 oxygen atoms. If we write O2 that means the oxygen molecule, which is two atoms of oxygen connected together. Sometimes people might write O2 to mean the same thing. If the number comes first, though, it has a different meaning. 2 O means 2 atoms of oxygen that aren't connected to anything.

- If we want to show how many protons and neutrons are present in an atom, we can use the mass number, as a superscript before the element symbol, such as13C. This means carbon with (protons + neutrons) = 13. You can tell that this is different from the charge, because the charge will always include + or – and come after the symbol.

- Most molecules or ions that are stable have an even number of electrons. If they have an odd number of electrons, this is called a radical. For instance, H is a radical, because it has one electron. Because this is unusual, it might be indicated with a dot, like this: H•. For instance, water is H2O, and if you remove hydrogen ion, you are left with hydroxide ion, OH–. If you remove H• from water, you are left with OH molecule, which is neutral. This is also called hydroxyl radical, written OH•.

- The phase of a substance is often indicated by a letter in () after the symbol. For instance, He is almost always a gas, written He(g). If it's a liquid (4.2K or below, less than -269° C) that is written He(l). You'll probably never hear about He(s), since it would be very hard to make it a solid. You might also see something written with (aq), which means "dissolved in water." For instance, NaCl(aq) means salt dissolved in water so there is no solid left. Or you might just see K+(aq), meaning potassium ions dissolved in water.
Elements
There are lots of elements and you don't need to memorize them all. Here are a few that you should learn right now, though, because they are common or important, so that you won't be confused when they are mentioned later. They are organized by their type.
- Non-metals
- Light elements: the elements with smallest mass
- Hydrogen (H): exists as H2 or in combination with other elements, such as in water
- Helium (He): named after the sun, because it was discovered in the sun before being discovered on Earth (we'll explain how later); it doesn't react with anything
- Major gases in air
- Oxygen (O): we get most of our energy from reactions with oxygen, when we breathe or when we burn fuel; O2 is 21% of air
- Nitrogen (N): often the limiting factor for agriculture or population growth, even though N2 is 78% of air, because it only reacts under special circumstances.
- Halogens: reactive elements that make salts; common negative ions
- Fluorine (F): the lightest halogen and most reactive element in the periodic table, people say that it killed the first two chemists who tried to isolate F2
- Chlorine (Cl): part of normal salt, NaCl, it is common in the ocean and in your body
- Bromine (Br): one of only two elements that are liquid at room temperature, bromine is also found in salts and minerals
- Iodine (I): a soft, shiny silver solid that easily evaporates to a purple gas, iodine can be used to disinfect cuts and is essential for human brains, suggesting that humans may have evolved to live near the ocean, which provides sources of iodine in fish and seaweed
- Main group solid non-metals: non-conductive and usually soft materials
- Carbon (C): the element on which biology is based, also found in diamond, graphite, coal, and charcoal
- Silicon (Si): the basis of the electronics industry; also a main component of sand, glass, and most rocks
- Sulfur (S): a smelly yellow solid, used to make strong acid in industry, also common in minerals and essential to life
- Phosphorus (P): first isolated from urine, although common in minerals; essential for life, often glows
- Metals: soft or hard, light or heavy, usually solid electric conductors
- Alkali metals: soft, light, common soluble positive ions
- Lithium (Li): the lightest alkali, used in batteries and anti-depressants
- Sodium (Na, you may know it as natrium): common in the ocean and salt
- Potassium (K, you may know it as kalium): also common, high concentration inside cells
- Alkaline Earth metals: like alkalis, but less so, less reactive, less soluble, positive ions more common in rocks, but also abundant in ocean
- Magnesium (Mg): common in rocks, essential for life, especially photosynthesis
- Calcium (Ca): common in biomaterials such as bone, tooth, and shells, also essential for muscles
- Main group metals
- Aluminum (Al, also called aluminium): requires lots of energy to produce the metal from the mineral sources, but very common and useful metal
- Tin (Sn, from Latin stannum): used since ancient times, especially in alloys such as bronze; still used in solder and many other applications
- Lead (Pb, from Latin plumbum): very heavy, soft, sweet-tasting toxic metal, commonly used since ancient times, now used to shield radiation and in bullets, among many other uses
- Transition Metals: a widely varied group, often characterized by complex chemical properties
- Iron (Fe, from Latin ferrum): most abundant element on earth, essential in steel, with complex reaction properties essential to life
- Copper (Cu, from Latin cuprum): less reactive metal, with characteristic colors, commonly used in coins and electronics
- Silver (Ag, from Latin argentum): used in jewelry, coins and other ornaments and utensils since ancient times, it didn't tarnish until after the industrial revolution, and now also used in electronics
- Gold (Au, from Latin aurum): used since ancient times in coins and jewelry, to color stained glass, also in dentistry and other applications
- Mercury (Hg, from Latin hydrargyrum): also called quicksilver, because it is a silver liquid, it is toxic but very important in the history of science; it may be familiar from thermometers
Common Positive Ions (Cations)
"[element name](charge in Roman numerals if needed) ion"
Cation is another word for positive ion. The common positive ions are the ions of the alkali and alkaline earth metals and ammonium, NH4+. The alkali metals form +1 cations, such as Na+ and K+. The alkaline earth metals form +2 cations, such as Ca2+ and Mg2+. The hydrogen ion, H+ is a very common cation. For these cations, you can call them "[element name] ion", such as sodium ion or calcium ion.
You'll also see transition metal cations or main group metal cations, but it is harder to predict what charge they will have, especially because some of them can have different charges, like iron, which is commonly Fe2+ or Fe3+. The charge on a transition metal cation can also be indicated using Roman numerals in parentheses, which looks like Fe(II) or Fe(III). The Roman numerals you will need to know for chemistry are:
| 1 |
2 |
3 |
3 |
5 |
6 |
7 |
8 |
9 |
10 |
| I |
II |
III |
IV |
V |
VI |
VII |
VIII |
IX |
X |
For cations that have uncertain charge, you should call them "[element name](charge in Roman numerals) ion." For instance, iron(II) ion or sometimes just Fe(II).
Sometimes people use special names for these ions, in which the higher charge ion is called "[name]-ic ion" and the lower charge ion is called "[name]-ous ion," such as ferrous for Fe(II) and ferric for Fe(III), or cuprous ion for Cu(I) and cupric ion for Cu(II). I think this is most common for Fe, and I've never heard anyone call nickel(II) nickelous ion because that sounds ridiculous.

Here's a list of common cations with less predictable charges:
Elements not on the list above, that you may see soon anyway: zinc(II): Zn2+, cadmium(II): Cd2+, cobalt(II): Co2+, manganese(II): Mn2+, nickel(II): Ni2+, chromium(III): Cr3+.
Common Negative Ions (Anions)
"[base name] + (-ide,-ate, or -ite)"
Anion is another word for negative ion. Common negative ions are the halide ions, formed from the halogen elements: fluoride, F–; chloride, Cl–; bromide, Br–; and iodide, I–. As you may have noticed, the names of anions have "-ide" at the end when they are formed from elements. Other examples include oxide, O2–, sulfide, S2–, and nitride, N3–.
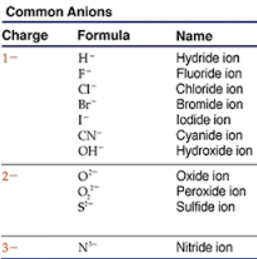
There are also many important polyatomic anions, which means anions that include more than one atom. These include toxic cyanide ion, CN–, common hydroxide ion, OH–, and peroxide ion, O22–. Other important anions include acetate ion (C2H3O2–), which is in vinegar, the chlorate ion (ClO3–), the perchlorate ion (ClO4–) which is often explosive, the nitrate ion (NO3–), the carbonate ion (CO32–) found in shells, the sulfate ion (SO42–), and the phosphate ion (PO43–). All of these end in "-ate", which means that they have more oxygen. Also, notice that "per-___-ate" means more oxygen than just "-ate", as in perchlorate.
Less common but still important are some "-ite" anions, which have less oxygen, such as nitrite (NO2–), sulfite (SO32–), chlorite (ClO2–) and hypochlorite (ClO–). Notice that "hypo-___-ite" means less oxygen than just "-ite" as in hypochlorite. Sulfite and nitrite are used to preserve foods. Sulfite salts are used in wine, dried fruit and preserved radish (mu). Nitrite salts are used in preserved meats.
One more rule says that if you take an anion like carbonate or sulfate and add one hydrogen ion, then you call that "bicarbonate" (HCO3–) or "bisulfate" (HSO4–). Or you might see it called "hydrogen carbonate" or "hydrogen sulfate." Note that because we added a hydrogen ion, the charge on the bicarbonate ion is one less than the charge on the carbonate ion. Also, note that "disulfate" is S2O72–, quite different from bisulfate.

Chemical Nomenclature for Ionic Compounds
"[cation name] + [anion name]"
Ionic compounds are compounds that include at least two components, a positive ion and a negative ion. Often the positive ion is a metal element ion and the negative ion is a non-metal ion. To name an ionic compound, you usually just give the cation followed by the anion, such as "sodium chloride" or "ammonium nitrate." If the cation is the type that could have different charges, than you should say what the charge is, such as "mercury(I) iodide" or "cupric sulfate."

Chemical Nomenclature for Acids
"(hydro if -ide)[anion base name] + (-ic if -ide, -ate; -ous if -ite) + acid"
Acid usually means an anion combined with the hydrogen ion as the cation. For instance, HCl is a common acid, which is the hydrogen ion and the chloride anion. If the anion ends in "-ide" then usually the acid is called "hydro-___-ic acid" such as "hydrochloric acid" for HCl. You'll see this for all the "hydrohalic acids" which are H + a halogen, such as "hydrofluoric acid" or "hydroiodic acid." You might also see "hydrocyanic acid" for HCN. If the anion ends in "-ate" than you call the acid "___-ic acid," such as "sulfuric acid," which is H2SO4, or "nitric acid." HNO3. If the anion ends in "-ite" than the acid name is "___-ous acid." such as "hypochlorous acid" for HClO. Notice that earlier "-ic" and "-ous" meant more and less charge for cations, such as ferric and ferrous ions of iron. Now it also means more and less oxygen in acids.

"(prefix, not mono)[less anion-like atom name] + (prefix)[more anion-like atom name]-ide"
Non-metal compounds are often called covalent compounds. They are named following a different rule from ionic compounds. You will need these "prefixes" which indicate how many of each type of atom are present:
| 1 |
2 |
3 |
3 |
5 |
6 |
7 |
8 |
9 |
10 |
| mono |
di |
tri |
tetra |
penta |
hexa |
hepta |
octa |
nona |
deca |
The prefixes come from Greek. You will put the element that is more left on the periodic table first, unless it is oxygen, which is always last unless it is in a compound with fluorine. This follows the same pattern as ionic compounds. In ionic compounds, the cation is written first, and you will notice that it is usually more to the left in the periodic table than the anion, which is written last. When you name covalent compounds, the atom that's more like an anion is written last. Fluorine is always most "anionic," and oxygen is next most "anionic," so they will always be last. (Fluorine is actually most electronegative, but we will study this concept much later, which is why right now I'm calling it "anion-like.") If both atoms are in the same group (same column of the periodic table) then the lower one is named first. Notice that the two most "anion-like," F and O, are in the upper right of the periodic table. The atom written second, that's more "anion-like" is named like an anion, with the "-ide" ending. For example, CO: carbon is on the left, so we can write "monocarbon monoxide." Actually people usually just call it "carbon monoxide." You can skip "mono" for the first element. For instance, SO3 is called "sulfur trioxide" and N2O4 is called "dinitrogen tetroxide." XeO2 is xenon dioxide, even though xenon is more to the right than oxygen, because oxygen is more like an anion than anything except fluorine. If the compound involves hydrogen, then you can leave out the prefixes, such as "hydrogen chloride" for HCl or "hydrogen sulfide" for H2S, because the numbers of each atom can be predicted as if it were an ionic substance. But actually many compounds of hydrogen have special names, such as "ammonia" for NH3, "methane" for CH4, "borane" for BH3, "silane" for SiH4 and "phosphine" for PH3. You should learn the first two of these now.

Summary
Key Info for Common Elements
| Element name |
Symbol |
Atomic number |
Commonly found as... |
| Hydrogen |
H |
1 |
H2(g), water (H2O), acid (H+(aq)) |
| Helium |
He |
2 |
He(g), He(l) if you want to make things very cold |
| Lithium |
Li |
3 |
Li+ always, (aq) or in solids with anions, lithium metal Li(s) only in chem class |
| Carbon |
C |
6 |
Covalent compounds, making 4 bonds |
| Nitrogen |
N |
7 |
N2(g) in air, in ammonia (NH3(g or l)), in basic covalent compounds, in proteins |
| Oxygen |
O |
8 |
O2(g) in air, in water, in rock and glass, usually combined with Si |
| Fluorine |
F |
9 |
F–(aq) or with cations in rock, in covalent compounds with carbon (non-stick pans) |
| Sodium (Natrium) |
Na |
11 |
Na+(aq) or with anions in salts, sodium metal (Na(s)) only in chem class |
| Magnesium |
Mg |
12 |
Mg2+(aq) or with anions in salts and rocks |
| Aluminum (Aluminium) |
Al |
13 |
Al3+ with anions in rocks and salts, industrially made Al(s) metal |
| Silicon |
Si |
14 |
Industrially made Si(s) in computer chips, Si(IV) oxides in sand, glass, most rocks |
| Phosphorus |
P |
15 |
Phosphates: PO43–, P2O74–, etc. in rock, DNA |
| Sulfur |
S |
16 |
S8(s), S2– or sulfate (SO42–) in salts or rocks |
| Chlorine |
Cl |
17 |
Cl–(aq) or with cations in salts, Cl2(g) or ClO–(aq) in disinfectants |
| Potassium (kalium) |
K |
19 |
K+(aq) or with anions in salts, potassium metal (K(s)) only in chem class |
| Calcium |
Ca |
20 |
Ca2+(aq) or with anions in salts and rocks |
| Iron |
Fe |
26 |
Fe(s) metal industrially made, Fe(II) or Fe(III) oxides or sulfides and other minerals |
| Copper |
Cu |
29 |
Natural Cu(s) metal, Cu(I) or Cu(II) salts or minerals, usually blue or green |
| Bromine |
Br |
35 |
Br–(aq) or with cations in salts |
| Silver |
Ag |
47 |
Natural Ag(s) metal, Ag(I) or Ag(II) in salts or sulfide minerals |
| Tin |
Sn |
50 |
Industrially made Sn(s) in alloys, Sn(II) or Sn(IV) salts and oxide or sulfide minerals |
| Iodine |
I |
53 |
I–(aq) or with cations in salts |
| Gold |
Au |
79 |
Natural Au(s) metal, rarely Au(I) or Au(III) salts |
| Mercury |
Hg |
80 |
Natural (but rare) metal Hg(l), Hg(II) sulfides and halides, Hg(I) exists as Hg22+ |
| Lead |
Pb |
82 |
Pb(s) in alloys, Pb(II) sulfide, carbonate and sulfate minerals, sometimes Pb(IV) salts or minerals |
Common Polyatomic Ions
| Name |
Formula |
Where you find it |
| Ammonium |
NH4+ |
Soluble salts, fertilizer |
| Cyanide |
CN– |
Toxic, in some plant products and dyes |
| Hydroxide |
OH– |
In bases and some minerals |
| Peroxide |
O22– |
In bleaches and disinfectants |
| Acetate |
C2H3O2– |
In vinegar |
| Perchlorate |
ClO4– |
Soluble salts and explosives, a strong acid |
| Chlorate |
ClO3– |
Similar to perchlorates but less stable |
| Chlorite |
ClO2– |
Disinfectants and bleaches, some explosive salts |
| Hypochlorite |
ClO– |
Disinfectants and bleaches, most salts unstable |
| Nitrate |
NO3– |
Soluble salts, fertilizer, explosives, a strong acid |
| Nitrite |
NO2– |
Preservatives for food |
| Carbonate |
CO32– |
In rock, seashells, cement; a base |
| Bicarbonate |
HCO3– |
A base (baking soda), in soda, in blood |
| Sulfate |
SO42– |
In salts, plaster, detergent, a strong acid |
| Bisulfate |
HSO4– |
Food additives |
| Sulfite |
SO32– |
Salts, food preservatives |
| Phosphate |
PO43– |
Salts, rock, fertilizers, a strong acid, in ATP and DNA |
Contributors and Attributions












