Learning Objectives
Make sure you thoroughly understand the following essential ideas:
- Define Avogadro's number and explain why it is important to know.
- Define the mole. Be able to calculate the number of moles in a given mass of a substance, or the mass corresponding to a given number of moles.
- Define molecular weight, formula weight, and molar mass; explain how the latter differs from the first two.
- Be able to find the number of atoms or molecules in a given weight of a substance.
- Find the molar volume of a solid or liquid, given its density and molar mass.
- Explain how the molar volume of a metallic solid can lead to an estimate of atomic diameter.
The chemical changes we observe always involve discrete numbers of atoms that rearrange themselves into new configurations. These numbers are HUGE— far too large in magnitude for us to count or even visualize, but they are still numbers, and we need to have a way to deal with them. We also need a bridge between these numbers, which we are unable to measure directly, and the weights of substances, which we do measure and observe. The mole concept provides this bridge, and is central to all of quantitative chemistry.
Counting Atoms: Avogadro's Number
Owing to their tiny size, atoms and molecules cannot be counted by direct observation. But much as we do when "counting" beans in a jar, we can estimate the number of particles in a sample of an element or compound if we have some idea of the volume occupied by each particle and the volume of the container. Once this has been done, we know the number of formula units (to use the most general term for any combination of atoms we wish to define) in any arbitrary weight of the substance. The number will of course depend both on the formula of the substance and on the weight of the sample. However, if we consider a weight of substance that is the same as its formula (molecular) weight expressed in grams, we have only one number to know: Avogadro's number.
Avogadro's number
Avogadro's number is known to ten significant digits:
\[N_A = 6.022141527 \times 10^{23}.\]
However, you only need to know it to three significant figures:
\[N_A \approx 6.02 \times 10^{23}. \label{3.2.1}\]
So \(6.02 \times 10^{23}\) of what? Well, of anything you like: apples, stars in the sky, burritos. However, the only practical use for \(N_A\) is to have a more convenient way of expressing the huge numbers of the tiny particles such as atoms or molecules that we deal with in chemistry. Avogadro's number is a collective number, just like a dozen. Students can think of \(6.02 \times 10^{23}\) as the "chemist's dozen".
Before getting into the use of Avogadro's number in problems, take a moment to convince yourself of the reasoning embodied in the following examples.
Example \(\PageIndex{1}\): Mass ratio from atomic weights
The atomic weights of oxygen and carbon are 16.0 and 12.0 atomic mass units (\(u\)), respectively. How much heavier is the oxygen atom in relation to carbon?
Solution
Atomic weights represent the relative masses of different kinds of atoms. This means that the atom of oxygen has a mass that is
\[\dfrac{16\, \cancel{u}}{12\, \cancel{u}} = \dfrac{4}{3} ≈ 1.33 \nonumber\]
as great as the mass of a carbon atom.
Example \(\PageIndex{2}\): Mass of a single atom
The absolute mass of a carbon atom is 12.0 unified atomic mass units (\(u\)). How many grams will a single oxygen atom weigh?
Solution
The absolute mass of a carbon atom is 12.0 \(u\) or
\[12\,\cancel{u} \times \dfrac{1.6605 \times 10^{–24}\, g}{1 \,\cancel{u}} = 1.99 \times 10^{–23} \, g \text{ (per carbon atom)} \nonumber\]
The mass of the oxygen atom will be 4/3 greater (from Example \(\PageIndex{1}\)):
\[ \left( \dfrac{4}{3} \right) 1.99 \times 10^{–23} \, g = 2.66 \times 10^{–23} \, g \text{ (per oxygen atom)} \nonumber\]
Alternatively we can do the calculation directly like with carbon:
\[16\,\cancel{u} \times \dfrac{1.6605 \times 10^{–24}\, g}{1 \,\cancel{u}} = 2.66 \times 10^{–23} \, g \text{ (per oxygen atom)} \nonumber\]
Example \(\PageIndex{3}\): Relative masses from atomic weights
Suppose that we have \(N\) carbon atoms, where \(N\) is a number large enough to give us a pile of carbon atoms whose mass is 12.0 grams. How much would the same number, \(N\), of oxygen atoms weigh?
Solution
We use the results from Example \(\PageIndex{1}\) again. The collection of \(N\) oxygen atoms would have a mass of
\[\dfrac{4}{3} \times 12\, g = 16.0\, g. \nonumber\]
Exercise \(\PageIndex{1}\)
What is the numerical value of \(N\) in Example \(\PageIndex{3}\)?
- Answer
-
Using the results of Examples \(\PageIndex{2}\) and \(\PageIndex{3}\).
\[N \times 1.99 \times 10^{–23} \, g \text{ (per carbon atom)} = 12\, g \nonumber\]
or
\[N = \dfrac{12\, \cancel{g}}{1.99 \times 10^{–23} \, \cancel{g} \text{ (per carbon atom)}} = 6.03 \times 10^{23} \text{atoms} \nonumber \]
There are a lot of atoms in 12 g of carbon.
Things to understand about Avogadro's number
- It is a number, just as is "dozen", and thus is dimensionless.
- It is a huge number, far greater in magnitude than we can visualize
- Its practical use is limited to counting tiny things like atoms, molecules, "formula units", electrons, or photons.
- The value of NA can be known only to the precision that the number of atoms in a measurable weight of a substance can be estimated. Because large numbers of atoms cannot be counted directly, a variety of ingenious indirect measurements have been made involving such things as Brownian motion and X-ray scattering.
- The current value was determined by measuring the distances between the atoms of silicon in an ultrapure crystal of this element that was shaped into a perfect sphere. (The measurement was made by X-ray scattering.) When combined with the measured mass of this sphere, it yields Avogadro's number. However, there are two problems with this:
- The silicon sphere is an artifact, rather than being something that occurs in nature, and thus may not be perfectly reproducible.
- The standard of mass, the kilogram, is not precisely known, and its value appears to be changing. For these reasons, there are proposals to revise the definitions of both NA and the kilogram.
Moles and their Uses
The mole (abbreviated mol) is the the SI measure of quantity of a "chemical entity", which can be an atom, molecule, formula unit, electron or photon. One mole of anything is just Avogadro's number of that something. Or, if you think like a lawyer, you might prefer the official SI definition:
Definition: The Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12
Avogadro's number (Equation \ref{3.2.1}) like any pure number, is dimensionless. However, it also defines the mole, so we can also express NA as 6.02 × 1023 mol–1; in this form, it is properly known as Avogadro's constant. This construction emphasizes the role of Avogadro's number as a conversion factor between number of moles and number of "entities".
Example \(\PageIndex{4}\): number of moles in N particles
How many moles of nickel atoms are there in 80 nickel atoms?
Solution
\[\dfrac{80 \;atoms}{6.02 \times 10^{23} \; atoms\; mol^{-1}} = 1.33 \times 10^{-22} mol \nonumber\]
Is this answer reasonable? Yes, because 80 is an extremely small fraction of \(N_A\).
Molar Mass
The atomic weight, molecular weight, or formula weight of one mole of the fundamental units (atoms, molecules, or groups of atoms that correspond to the formula of a pure substance) is the ratio of its mass to 1/12 the mass of one mole of C12 atoms, and being a ratio, is dimensionless. But at the same time, this molar mass (as many now prefer to call it) is also the observable mass of one mole (NA) of the substance, so we frequently emphasize this by stating it explicitly as so many grams (or kilograms) per mole: g mol–1.
It is important always to bear in mind that the mole is a number and not a mass. But each individual particle has a mass of its own, so a mole of any specific substance will always correspond to a certain mass of that substance.
Example \(\PageIndex{5}\): Boron content of borax
Borax is the common name of sodium tetraborate, \(\ce{Na2B4O7}\).
- how many moles of boron are present in 20.0 g of borax?
- how many grams of boron are present in 20.0 g of borax?
Solution
The formula weight of \(\ce{Na2B4O7}\) so the molecular weight is:
\[(2 \times 23.0) + (4 \times 10.8) + (7 \times 16.0) = 201.2 \nonumber\]
- 20 g of borax contains (20.0 g) ÷ (201 g mol–1) = 0.10 mol of borax, and thus 0.40 mol of B.
- 0.40 mol of boron has a mass of (0.40 mol) × (10.8 g mol–1) = 4.3 g.
Example \(\PageIndex{6}\): Magnesium in chlorophyll
The plant photosynthetic pigment chlorophyll contains 2.68 percent magnesium by weight. How many atoms of Mg will there be in 1.00 g of chlorophyll?
Solution
Each gram of chlorophyll contains 0.0268 g of Mg, atomic weight 24.3.
- Number of moles in this weight of Mg: (.0268 g) / (24.2 g mol–1) = 0.00110 mol
- Number of atoms: (0.00110 mol) × (6.02E23 mol–1) = \(6.64 \times 10^{20}\)
Is this answer reasonable? (Always be suspicious of huge-number answers!) Yes, because we would expect to have huge numbers of atoms in any observable quantity of a substance.
Molar Volume
This is the volume occupied by one mole of a pure substance. Molar volume depends on the density of a substance and, like density, varies with temperature owing to thermal expansion, and also with the pressure. For solids and liquids, these variables ordinarily have little practical effect, so the values quoted for 1 atm pressure and 25°C are generally useful over a fairly wide range of conditions. This is definitely not the case with gases, whose molar volumes must be calculated for a specific temperature and pressure.
Example \(\PageIndex{7}\): Molar Volume of a Liquid
Methanol, CH3OH, is a liquid having a density of 0.79 g per milliliter. Calculate the molar volume of methanol.
Solution
The molar volume will be the volume occupied by one molar mass (32 g) of the liquid. Expressing the density in liters instead of mL, we have
\[V_M = \dfrac{32\; g\; mol^{–1}}{790\; g\; L^{–1}}= 0.0405 \;L \;mol^{–1} \nonumber\]
The molar volume of a metallic element allows one to estimate the size of the atom. The idea is to mentally divide a piece of the metal into as many little cubic boxes as there are atoms, and then calculate the length of each box. Assuming that an atom sits in the center of each box and that each atom is in direct contact with its six neighbors (two along each dimension), this gives the diameter of the atom. The manner in which atoms pack together in actual metallic crystals is usually more complicated than this and it varies from metal to metal, so this calculation only provides an approximate value.
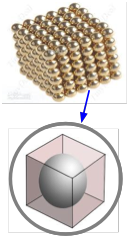
Example \(\PageIndex{8}\): Radius of a Strontium Atom
The density of metallic strontium is 2.60 g cm–3. Use this value to estimate the radius of the atom of Sr, whose atomic weight is 87.6.
Solution
The molar volume of Sr is:
\[\dfrac{87.6 \; g \; mol^{-1}}{2.60\; g\; cm^{-3}} = 33.7\; cm^3\; mol^{–1}\]
The volume of each "box" is"
\[\dfrac{33.7\; cm^3 mol^{–1}} {6.02 \times 10^{23}\; mol^{–1}} = 5.48 \times 10^{-23}\; cm^3\]
The side length of each box will be the cube root of this value, \(3.79 \times 10^{–8}\; cm\). The atomic radius will be half this value, or
\[1.9 \times 10^{–8}\; cm = 1.9 \times 10^{–10}\; m = 190 pm\]
Note: Your calculator probably has no cube root button, but you are expected to be able to find cube roots; you can usually use the xy button with y=0.333. You should also be able estimate the magnitude of this value for checking. The easiest way is to express the number so that the exponent is a multiple of 3. Take \(54 \times 10^{-24}\), for example. Since 33=27 and 43 = 64, you know that the cube root of 55 will be between 3 and 4, so the cube root should be a bit less than 4 × 10–8.
So how good is our atomic radius? Standard tables give the atomic radius of strontium is in the range 192-220 pm.


