4.1: Introduction
- Page ID
- 46562
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)And so, nothing that to our world appears,
Perishes completely, for nature ever
Upbuilds one thing from another's ruin;
Suffering nothing yet to come to birth
But by another's death.
Lucretius (95-55 B.C.)
Introduction
The main question asked in Chapter 2 was "If a given set of substances will react to give a desired product, how much of each substance is needed?" Our basic assumptions were that matter cannot be arbitrarily created or destroyed, and that atoms going into a reaction must come out again as products.
In this chapter we ask a second question: "Will a reaction occur, eventually?" Is there a tendency or a drive for a given reaction to take place, and if we wait long enough will we find that reactants have been converted spontaneously into products? This question leads to the ideas of spontaneity and of chemical equilibrium. A third question, "Will a reaction occur in a reasonably short time?" involves chemical kinetics, which will be discussed in Chapter 22. For the moment, we will be satisfied if we can predict which way a chemical reaction will go by itself, ignoring the time factor.
Spontaneous Reactions
A chemical reaction that will occur on its own, given enough time, is said to be spontaneous. In the open air, and under the conditions inside an automobile engine, the combustion of gasoline is spontaneous:
C7H16 + 11 O2 → 7CO2 + 8 H2O
(The reaction is exothermic, or heat emitting. The enthalpy change, which was defined in Chapter 2, is large and negative: 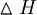 = -4812 kJ mole-1 of heptane at 298 K. The heat emitted causes the product gases to expand, and it is the pressure from these expanding gases that drives the car.) In contrast, the reverse reaction under the same conditions is not spontaneous:
= -4812 kJ mole-1 of heptane at 298 K. The heat emitted causes the product gases to expand, and it is the pressure from these expanding gases that drives the car.) In contrast, the reverse reaction under the same conditions is not spontaneous:
7CO2 + 8H2OC7H16 + 11 O2
No one seriously proposes that gasoline can be obtained spontaneously from a mixture of water vapor and carbon dioxide.
Explosions are examples of rapid, spontaneous reactions, but a reaction need not be as rapid as an explosion to be spontaneous. It is important to understand clearly the difference between rapidity and spontaneity. If you mix oxygen and hydrogen gases at room temperature, they will remain together without appreciable reaction for years. Yet the reaction to produce water is genuinely spontaneous:
2H2 + O2 → 2H2O
We know that this is true because we can trigger the reaction with a match, or catalyst of finely divided platinum metal.
The preceding sentence suggests why a chemist is interested in whether a reaction is spontaneous, that is, whether it has a natural tendency to occur. If a desirable chemical reaction is spontaneous but slow, it may be possible to speed up the process. Increasing the temperature will often do the trick, or a catalyst may work. We will discuss the functions of a catalyst in detail in Chapter 22. But in brief, we can say now that a catalyst is a substance that helps a naturally spontaneous reaction to go faster by providing an easier pathway for it. Gasoline will burn rapidly in air at a high enough temperature. The role of a spark plug in an automobile engine is to provide this initial temperature. The heat produced by the reaction maintains the high temperature needed to keep it going thereafter. Gasoline will combine with oxygen at room temperature if the proper catalyst is used, because the reaction is naturally spontaneous but slow. But no catalyst will ever make carbon dioxide and water recombine to produce gasoline and oxygen at room temperature and moderate pressures, and it would be a foolish chemist who spent time trying to find such a catalyst. In short, an understanding of spontaneous and nonspontaneous reactions helps a chemist to see the limits of what is possible. If a reaction is possible but not currently realizable, it may be worthwhile to look for ways to carry it out. If the process is inherently impossible, then it is time to study something else.
Equilibrium and the Equilibrium Constant
The speed with which a reaction takes a place ordinarily depends on the most concentrations of the reacting substances. This is common sense, since most reactions take place when molecules collide, and the more molecules there are per unit of volume, the more often collisions will occur.
The industrial fixation of atmospheric nitrogen is very important in the manufacture of agricultural fertilizers (and explosives). One of the steps in nitrogen fixation, in the presence of a catalyst, is
N2 + O2 → 2NO (4-1)
If this reaction took place by simple collision of one molecule of N2 and one molecule of O2, then we would expect the rate of collision (and hence the rate of reaction) to be proportional to the concentrations of N2 and O2:
Rate of NO production![\textstyle\propto \!\, [N_2][O_2]](https://upload.wikimedia.org/math/e/c/c/ecc56b5fd446904b3808388f689e307b.png)
or
R1 = k1[N2][O2] (4-2)
The proportionality constant k1 is called the forward-reaction rate constant, and the bracketed terms [N2][O2] represent concentrations in moles per liter. This rate constant, which we will discuss in more detail in Chapter 22, usually varies with temperature. Most reactions go faster at higher temperatures, so k1 is larger at higher temperatures. But k1 does not depend on the concentrations of nitrogen and oxygen gases present. All of the concentration dependence of the overall forward reaction rate, R1, is contained in the terms [N2] and [O2]. If this reaction began rapidly in a sealed tank with high starting concentrations of both gases, then as more N2 and O2 were consumed, the forward reaction would become progressively slower. The rate of reaction would decrease because the frequency of collision of molecules would diminish as fewer N2 and O2 molecules were left in the tank.
The reverse reaction can also occur. If this reaction took place by the collision of two molecules of NO to make one molecule of each starting gas,
2NO → N2 + O2 (4-3)
then the rate of reaction again would be proportional to the concentration of each of the colliding molecules. Since these molecules are of the same compound, NO, the rate would be proportional to the square of the NO concentration:
Rate of NO removal[NO][NO]
or
R2 = k2[NO]2 (4-4)
where R2 is the overall reverse reaction rate and k2 is the rev~rse-reaction rate constant. If little NO is present when the experiment begins, this reaction will occur at a negligible rate. But as more NO accumulates by the forward reaction, the faster it will be broken down by the reverse reaction.
Thus as the forward rate, R1, decreases, the reverse rate, R2 , increases. Eventually the point will be reached at which the forward and reverse reactions exactly balance (4-5):
-
-
R1 = R2 [N2][O2]k1 = k2[NO]2
-
This is the condition of equilibrium. Had you been monitoring the concentrations of the three gases, N2 O2, and NO, you would have found that the composition of the reacting mixture had reached an equilibrium state and thereafter ceased to change with time. This does not mean that the individual reactions had stopped, only that they were proceeding at equal rates; that is, they had arrived at, and thereafter maintained, a condition of balance or equilibrium.

The condition of equilibrium can be illustrated by imagining two large fish tanks, connected by a channel (Figure 4-1). One tank initially contains 10 goldfish, and the other contains 10 guppies. If you watch the fish swimming aimlessly long enough, you will eventually find that approximately 5 of each type of fish are present in each tank. Each fish has the same chance of blundering through the channel into the other tank. But as long as there are more goldfish in the left tank (Figure 4-la), there is a greater probability that a goldfish will swim from left to right than the reverse. Similarly, as long as the number of guppies in the right tank exceeds that in the left, there will be a net flow of guppies to the left, even though there is nothing in the left tank to make the guppies prefer it. Thus the rate of flow of guppies is proportional to the concentration of guppies present. A similar statement can be made for the goldfish.
At equilibrium (Figure 4-1b), on an average there will be 5 guppies and 5 goldfish in each tank. But they will not always be the same 5 of each fish. If 1 guppy wanders from the left tank into the right, then it or a different guppy may wander back a little later. Thus at equilibrium we find that the fish have not stopped swimming, only that over a period of time the total number of guppies and goldfish in each tank remains constant. If we were to fill each tank with 9 goldfish and then throw in 1 guppy, we would see that, in its aimless swimming, it would spend half its time in one tank and half in the other (Figure 4-1 c).
In the NO reaction we considered, there will be a constant concentration of NO molecules at equilibrium, but they will not always be the same NO molecules. Individual NO molecules will react to re-form N2 and O2, and other reactant molecules will make more NO. As with the goldfish, only on a head-count or concentration basis have changes ceased at equilibrium.
The equilibrium condition for the NO-producing reaction, equation 4-1, can be rewritten in a more useful form:
(4-6)
in which the ratio of forward and reverse rate constants is expressed as a simple constant, the equilibrium constant, Keq. This equilibrium constant will vary as the temperature varies, but it is independent of the concentrations of the reactants and products. It tells us the ratio of products to reactants at equilibrium, and is an extremely useful quantity for determining whether a desired reaction will take place spontaneously.
General Form of the Equilibrium Constant
We derived the equilibrium-constant expression for the NO reaction by assuming that we knew the way that the forward and reverse steps occurred at the molecular level. If the NO reaction proceeded by simple collision of two molecules, the derivation would be perfectly correct. The actual mechanism of this reaction is more complicated. But it is important, and fortunate for chemists, that we do not have to know the reaction mechanism to write the proper equilibrium constant. The equilibrium-constant expression can always be written from the balanced chemical equation, with no other information, even when the forward and reverse rate expressions are more complicated than the balanced equation would suggest. (We shall prove this in Chapter 16.) In our NO example, the forward reaction actually takes place by a series of complicated chain steps. The reverse reaction takes place by a complementary set of reactions, so that these complications cancel one another in the final ratio of concentrations that gives us the equilibrium constant. The details of the mechanism are "invisible" to the equilibrium-constant expression, and irrelevant to equilibrium calculations.
A general chemical reaction can be written as
aA + bBcC + dD (4-7)
In this expression, A and B represent the reactants; C and D, the products. The letters a, b, c, and d represent the number of moles of each substance involved in the balanced reaction, and the double arrows indicate a state of equilibrium. Although only two reactants and two products are shown in the general reaction, the principle is extendable to any number. The correct equilibrium-constant expression for this reaction is
(4-8)
It is the ratio of product concentrations to reactant concentrations, with each concentration term raised to a power given by the number of moles of that substance appearing in the balanced chemical equation. Because it is based on the quantities of reactants and products present at equilibrium, equation 4-8 is called the law of mass action.
|
Give the equilibrium-constant expression for the reaction
|
|
Solution The equilibrium constant is given by |
Since all four substances have a coefficient of 1 in the balanced equation, their concentrations are all raised to the first power in the equilibrium-constant expression.
|
What is the equilibrium-constant expression for the formation of water from hydrogen and oxygen gases? The reaction is
|
|
Solution |
Since two moles of hydrogen and water are involved in the chemical equation, their concentrations are squared in the Keq expression.
|
Give the equilibrium-constant expression for the dissociation (breaking up) of water into hydrogen and oxygen. The reaction is
|
|
Solution |
An important general point emerges here. This reaction is the reverse of that of Example 2, and the equilibrium-constant expression is the inverse, or reciprocal, of the earlier one. If a balanced chemical reaction is reversed, then the equilibrium-constant expression must be inverted, since what once were reactants now are products, and vice versa.
|
The dissociation of water can just as properly be written as
What then is the equilibrium-constant expression? |
|
Solution |
Notice that when the reaction from Example 3 is divided by 2, resulting in the Example 4 reaction, the equilibrium constant is the square root of the old value, or the old Keq to the one-half power. Similarly, if the reaction is doubled, the Keq must be squared. In general, it is perfectly proper to multiply all the coefficients of a balanced chemical reaction by any positive or negative number, n, and the equation will remain balanced. (Multiplying all the coefficients of an equation by - 1 is formally the same as writing the equation in reverse. Write out a simple equation and prove to yourself that this is so.) But if all the co1ficients of an equation are multiplied by n, then the new equilibrium-constant expression is the old one raised to the nth power. Hence, when working with equilibrium constants, one must keep the corresponding chemical reactions clearly in mind.
|
The reaction for the formation or the breakdown of ammonia can be written in a number of ways:
(Each of these expressions might be appropriate, depending on whether you were focusing on nitrogen, ammonia, hydrogen, or the dissociation of ammonia.) What are the equilibrium-constant expressions for each formulation, and how are the equilibrium constants related? |
|
Solution
|
Notice that there is nothing wrong with fractional powers in the equilibrium-constant expression.
Using Equilibrium Constants
Equilibrium constants have two main purposes:
-
- 1. To help us tell whether a reaction will be spontaneous under specified conditions.
- 2. To enable us to calculate the concentration of reactants and products that will be present once equilibrium has been reached.
We can illustrate how equilibrium constants can be used to achieve these ends, and also the fact that an equilibrium constant is indeed constant, with real data from one of the most intensively studied of all reactions, that between hydrogen and iodine to yield hydrogen iodide:
H2(g) + I2(g)2HI(g) (4-9)

If we mix hydrogen and iodine in a sealed flask and observe the reaction, the gradual fading of the purple color of the iodine vapor tells us that iodine is being consumed. This reaction was studied first by the German chemist Max Bodenstein in 1893. Table 4-1 contains the data from Bodenstein's experiments. The experimental data are in the first three columns. In the fourth column, we have calculated the simple ratio of product and reactant concentrations, [HI]/[H2][I2], to see if it is constant. It clearly is not, for as the hydrogen concentration is decreased and the iodine concentration is increased, this ratio varies from 2.60 to less than 1. The law of mass action (Section 4-3) dictates that the equilibrium-constant expression should contain the square of the HI concentration, since the reaction involves 2 moles of HI for every mole of H2 and I2 , The fifth column shows that the ratio [HI]2/[H2][I2] is constant within a mean deviation of approximately 3%. * Therefore, this ratio is the proper equilibrium-constant expression, and the average value of Keq for these six runs is 50.53.
The equilibrium constant can be used to determine whether a reaction under specified conditions will go spontaneously in the forward or in the reverse direction. The ratio of product concentration to reactant concentration, identical to the equilibrium constant in form but not necessarily at equilibrium conditions, is called the reaction quotient, Q:
Q =(not necessarily at equilibrium) (4-10)
If there are too many reactant molecules present for equilibrium to exist, then the concentration terms in the denominator will make the reaction quotient, Q, smaller than Keq. The reaction will go forward spontaneously to make more product. However, if an experiment is set up so that the reaction quotient is greater than Keq, then too many product molecules are present for equilibrium and the reverse reaction will proceed spontaneously. Therefore, a comparison of the actual concentration ratio or reaction quotient with the equilibrium constant allows us to predict in which direction a reaction will go spontaneously under the given set of circumstances:
Q < Keq (forward reaction spontaneous)
Q > Keq (reverse reaction spontaneous) (4-11)
Q = Keq (reactants and products at equilibrium)
- These are Bodenstein's original numbers. Modern data can be much more accurate, with
less deviation in Keq. The mean deviation is the average of the deviations of individual calculated Keq from the average Keq.
| If 1.0 X 10-2 mole each of hydrogen and iodine gases are placed in a I-liter flask at 448°C with 2.0 X 10-3 mole of HI, will more HI be produced? |
|
Solution The reaction quotient under these conditions is
This is smaller than the equilibrium value of 50.53 in Table 4-1, which tells us that excess reactants are present. Hence, equilibrium will not be reached until more HI has been formed. |
| If only 1.0 X 10-3 mole each of H2 and I2 had been used, together with 2.0 X 10-3 mole of HI, would more HI have been produced spontaneously? |
|
Solution You can verify that the reaction quotient is Q = 4.0. Because this is less than Keq, the forward reaction is still spontaneous. |
| If the conditions of Example 7 are changed so that the HI concentration is increased to 2.0 X 10-2 mole liter-1 , what happens to the reaction? |
|
Solution The reaction quotient now is Q = 400. This is greater than Keq- There are now too many product molecules and too few reactant molecules for equilibrium to exist. Thus the reverse reaction occurs more rapidly than the forward reaction. Equilibrium is reached only by converting some of the HI to H2 and 12, so the reverse reaction is spontaneous. |
| If the conditions of Example 7 are changed so that the HI concentration is 7.1 X 10-3 mole liter-1 , in which direction is the reaction spontaneous? |
|
Solution Under these conditions,
Since Q equals Keq within the limits of accuracy of the data, the system as described is at equilibrium, and neither the forward nor the backward reaction is spontaneous. (Both reactions are still taking place at the molecular level, of course, but they are balanced so their net effects cancel.) |
The second use for equilibrium constants is to calculate the concentrations of reactants and products that will be present at equilibrium.
| If a 1-liter flask contains 1.0 X 10-3 mole each of H2 and I2 at 448°C, what amount of HI is present when the gas mixture is at equilibrium? |
|
Solution The Keq expression is treated as an ordinary algebraic equation, and solved for the HI concentration:
You can verify that in Example 7 the HI concentration was less than this equilibrium value; in Example 8 it was more; and in Example 9 it was just this value. |
| One-tenth of a mole, 0.10 mole, of hydrogen iodide is placed in an otherwise empty 5.0 liter flask at 448°C. When the contents have come to equilibrium, how much hydrogen and iodine will be in the flask? | ||||||||||||
|
Solution From the stoichiometry of the reaction, the concentrations of H2 and I2 must be the same. For every mole of H2 and I2 formed, 2 moles of HI must decompose. Let y equal the number of moles of H2 or I2 per liter present at equilibrium. The initial concentration of HI before any dissociation has occurred is
Begin by writing a balanced equation for the reaction, then make a table of concentrations at the start and at equilibrium:
The HI concentration of 0.020 mole liter-1 has been decreased by 2y for every y moles of H2 and I2 that are formed. The equilibrium-constant expression is
We immediately see that we can take a shortcut by taking the square root of both sides:
For 5 liters, 5
|
In many cases a quadratic equation must be solved.
| If 0.00500 mole of hydrogen gas and 0.0100 mole of iodine gas are placed in a 5.00 liter tank at 448°C, how much HI will be present at equilibrium? | |||||||||||||||||||||||||||
|
Solution The initial concentrations of H2 and I2 are
This time, let the unknown variable y be the moles per liter of H2 or I2 that have reacted at equilibrium:
The quilibrium expression is
The square-root shortcut is now impossible because the starting concentrations of H2 and I2 are unequal. Instead we must reduce the equation to a quadratic expression:
A general quadratic equation of the form ay2 + by + c = 0 can be solved by the quadratic formula,
Thus for this problem
The first solution is physically impossible since it shows more H2 reacting than was originally present. The second solution is the correct answer: y=0.935
|
Units and Equilibrium Constants
As we have seen, the square brackets around a chemical symbol, as in [N2], represent concentrations, usually but not exclusively in units of moles liter-1. Concentrations expressed as moles liter-1 are often given the special symbol c, as in cN2, the concentrations measured in these units is denoted by Kc.
An equilibrium constant as we have defined it thus far may itself have units. In Example 1, Keq is unitless since the moles2 1iter-2 of the numerator and denominator cancel. In Example 2, the units of Keq are moles-1 liter since concentration occurs to the second power in the numerator and to the third power in the denominator. In Example 3 the units of Keq are the inverse: moles liter-1. The units demanded by Example 4, moles1/2 liter-1/2, may seem strange but they are perfectly respectable.
| What are the units for the equilibrium constants in the four reactions of Example 5? | ||||||||||||||||||||
|
Solution The Keq expression is treated as an ordinary algebraic equation, and solved for the HI concentration:
|
|
One step in the commercial synthesis of sulfuric acid is the reaction of sulfur dioxide and oxygen to make sulfur trioxide:
At 1000 K, the equilibrium constant for this reaction is Kp = 3.50 atm-1. If the total pressure in the reaction chamber is 1.00 atm and the partial pressure of unused 02 at equilibrium is 0.10 atm, what is the ratio of concentrations of product (S03) to reactant (S02)? |
|
Solution
The equilibrium mixture has 0.59 mole of S03 for every 1 mole of S02. |
The ideal gas law permits us to convert between atmospheres and moles liter-1, and between Kp and Kc:
PV = nRT (3-8)
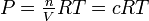 (4-12)
(4-12)
In the general chemical reaction written earlier,
aA + bBcC + dD (4-7)
Δn (read "delta n"), the increase in number of moles of gas during the reaction, is
 n = c + d - a - b (4-13)
n = c + d - a - b (4-13)
The equilibrium-constant expression in terms of partial pressures is
(4-14)
With the ideal gas law applied to each gas component, we can convert this expression to Kc:
(RT)Δn = Kc(RT)Δn (4-15)
(Do not confuse the two uses of the symbol c in equation 4-15: one is for concentration in moles liter-1 and the other for the number of moles of substance C.)
| What is the numerical value of Kc for the reaction of Example 14? |
|
Solution Three moles of reactant gases are converted into only 2 moles of product, so Δn = - 1. Hence at 1000 K,
|
Although the numerical answers that result when different units are used may differ, the physical reality must be the same.
| What is the concentration of oxygen in Example 14, in moles liter-1? Solve Example 14 again using Kc from Example 15. |
|
Solution Three moles of reactant gases are converted into only 2 moles of product, so Δn = - 1. Hence at 1000 K,
This is the same ration of SO_3 to SO_4 as was obtained when atmospheres were used. The choice is one of convenience. |
Equilibrium Involving Gases with Liquids or Solids

All the examples considered so far have involved only one physical state, a gas, and are examples of homogeneous equilibria. Equilibria that involve two or more physical states (such as a gas with a liquid or a solid) are called hetergenous equilibria. If one or more of the reactants or products are solids or liquids, how does this affect the form of the equilibrium constant?
The answer, in short, is that any pure solids or liquids that may be present at equilibrium have the same effect on the equilibrium no matter how much solid or liquid is present. The concentration of a pure solid or liquid can be considered constant, and for convenience all such constant terms are brought to the left side of the equation and incorporated into the equilibrium constant itself. As an example, limestone (calcium carbonate, CaCO3), breaks down into quicklime (calcium oxide, CaO) and carbon dioxide, CO2:
-
- CaCO3
 CaO(s) + CO2(g)
CaO(s) + CO2(g)
- CaCO3
The simple equilibrium-constant expression is
-
- K'eq =
![\textstyle\frac{[CaO(s)][CO_2(g)]}{[CaCO_3(s)]}](https://upload.wikimedia.org/math/1/d/9/1d9983f0ee9f4c65fa2bbf91a455e0e9.png)
- K'eq =
As long as any solid limestone and quicklime are in contact with the gas, their effect on the equilibrium is unchanging. Hence the terms [CaCO3] and [CaO] remain constant and can be merged with K'eq:
-
- Keq = K'eq
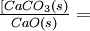 [CO2(g)]
[CO2(g)]
- Keq = K'eq
This form of the equilibrium-constant expression tells us that, at a given temperature, the concentration of carbon-dioxide gas above limestone and calcium oxide is a fixed quantity. (this is true only as long as both solid forms are present.) Measuring concentration in units of atmospheres, we get
-
- Kp = pCO2
with the experimental value 0.236 atm at 800°C.
We can see what this means experimentally by considering a cylinder to which CaCO, and CaO have been added. The cylinder has a movable piston, as shown in Figure 4-2. If the piston is fixed at one position, then CaCO3 will decompose until the pressure of CO2 above the solids is 0.236 atm (if the temperature is 800°C). If you try to decrease the pressure by raising the piston, then more CaCO3 will decompose until the pressure again rises to 0.236 atm. Conversely, if you try to increase the pressure by lowering the piston, some of the CO2 gas will react with CaO and become CaCO3 decreasing the amount of CO2 gas present until the pressure once more is 0.236 atm. The only way to increase pCO2, is to raise the temperature, which increases the value of Kp itself to 1 atm at 894°C and to 1.04 atm at 900°C.
An even simpler example is the vaporization of a liquid such as water:
-
- H2O(l)
 H2O(g)
H2O(g)
- H2O(l)
This process can be treated as a chemical reaction in a formal sense even though bonds within molecules are not made or broken. Imagine that the cylinder shown in Figure 4-2 is half-filled with water rather than with CaCO3 and CaO, and that the piston is initially brought down to the surface of the water. As the piston is raised, liquid will evaporate until the pressure of water vapor is a constant value that depends only on the temperature. This is the equilibrium vapor pressure of water at that temperature. At 25°C, the vapor pressure of water is 0.0313 atm. At 100°C, the vapor pressure reaches 1 atm and, as we shall see in Chapter 18, this is just the definition of the normal boiling point of water. The pressure of water vapor above the liquid in the cylinder does not depend on whether the water in the cylinder is 1 cm or 10 cm deep; the only requirement is that some water be present and capable of evaporating to make up any decrease in vapor pressure. Only when the piston is raised to the point where no more liquid exists can the pressure of water vapor fall below 0.0313 atm, if the cylinder is at 25°C. Similarly, if the piston is lowered, some of the vapor condenses, keeping the pressure at 0.0313 atm. Only when all vapor has condensed and the piston is resting on the surface of the liquid can the pressure inside the cylinder be raised above 0.0313 atm.
The formal equilibrium treatment of the evaporation of water would be
-
- K'eq =
![\textstyle\frac{[H_2O(g)]}{[H_2O(l)]}](https://upload.wikimedia.org/math/1/c/0/1c01d498a08bee5cc2f0b20feb722c95.png)
- K'eq =
- [H2O(l)] = constant , as long as liquid is present
-
- Keq = K'eq[H2O(l)] = [H2O(g)]
In pressure units, the expression would be
-
- Kp = pH2O(g)
From a practical standpoint, what the preceding discussion means is that the concentration terms for pure solids and liquids are simply eliminated from the equilibrium-constant expression. (They are present, implicitly, in the Keq.)
| If the hydrogen iodide reaction previously discussed in this chapter is carried out at room temperature, then iodine is present as deep purple crystals rather than as vapor. What then is the form of the equilibrium-constant expression, and does the equilibrium depend on the amount of iodine crystals present? |
|
Solution The reaction is
and the equilibrium-constant expression is:
As long as some I2(s) crystals are present, the quantity is immaterial as far as equilibrium is concerned. |
|
Tin(IV) oxide reacts with carbon monoxide to form metallic tin and CO2 by the reaction
What is the equilibrium-constant expression? |
|
Solution
|
|
What is the equilibrium-constant expression for the following reaction leading to liquid water?
What would the expression be if the product were water vapor? |
|
Solution If the product is H2O(l), the equilibrium-constant expression is
If the product is H2O(g), the equilibrium-constant expression is
|
The preceding example shows that as long as liquid water is present the gas-phase concentration is fixed at the vapor pressure of water at that temperature. Hence the water contribution, being constant, can be lumped into Keq.
Factors Affecting Equilibrium: Le Chatelier's Principle
Equilibrium represents a balance between two opposing reactions. How sensitive is this balance to changes in the conditions of a reaction? What can be done to change the equilibrium state? These are very practical questions if, for example, one is trying to increase the yield of a useful product in a reaction.
Under specified conditions, the equilibrium-constant expression tells us the ratio of product to reactants when the forward and backward reactions are in balance. This equilibrium constant is not affected by changes in concentration of reactants or products. However, if products can be withdrawn continuously, then the reacting system can be kept constantly off-balance, or short of equilibrium. More reactants will be used and a continuous stream of new products will be formed. This method is useful when one product of the reaction can escape as a gas, be condensed or frozen out of a gas phase as a liquid or solid, be washed out of the gas mixture by a spray of a liquid in which it is especially soluble, or be precipitated from a gas or solution.
For example, when solid lime (CaO) and coke (C) are heated in an electric furnace to make calcium carbide (CaC2),
-
- CaO(s) + 3C(s)
 CaC2(s) + CO(g)↑
CaC2(s) + CO(g)↑
- CaO(s) + 3C(s)
the reaction, which at 2000-3000°C has an equilibrium constant of close to 1.00, is tipped toward calcium carbide formation by the continuous removal of carbon monoxide gas. In the industrial manufacture of titanium dioxide for pigments, TiCl4 and O2 react as gases:
-
- TiCl4 (g) + O2 (g)
 TiO2 (s)↓ + 2Cl2(g)
TiO2 (s)↓ + 2Cl2(g)
- TiCl4 (g) + O2 (g)
The product separates from the reacting gases as a fine powder of solid Ti02 , and the reaction is thus kept moving in the forward direction. When ethyl acetate or other esters used as solvents and flavorings are synthesized from carboxylic acids and alcohols,
-
- CH2COOH + HOCH2CH3
 CH3COOCH2CH3 + H2O
CH3COOCH2CH3 + H2O - acetic acid ethanol ethyl acetate
- CH2COOH + HOCH2CH3
the reaction is kept constantly off-balance by removing the water as fast as it is formed. This can be done by using a drying agent such as Drierite (CaS04), by running the reaction in benzene and boiling off a constant-boiling benzene-water mixture, or by running the reaction in a solvent in which the water is completely immiscible and separates as droplets in a second phase. A final example: Since ammonia is far more soluble in water than either hydrogen or nitrogen is, the yield of ammonia in the reaction
-
- N2(g) + 3H2(g)
 2NH3(g)
2NH3(g)
- N2(g) + 3H2(g)
can be raised to well over 90% by washing the ammonia out of the equilibrium mixture of gases with a stream of water, and recycling the nitrogen and hydrogen.
Temperature

All the preceding methods will upset an equilibrium (in our examples, in favor of desired products) without altering the equilibrium constant. A chemist can often enhance yields of desired products by increasing the equilibrium constant so that the ratio of products to reactants at equilibrium is larger. The equilibrium constant is usually temperature dependent. In general, both forward and reverse reactions are speeded up by increasing the temperature, because the molecules move faster and collide more often. If the increase in the rate of the forward reaction is greater than that of the reverse, then Keq. increases with temperature and more products are formed at equilibrium. If the reverse reaction is favored, then Keq. decreases. Thus Keq for the hydrogen- iodine reaction at 448°C is 50.53, but at 425°C it is 54.4, and at 357°C it increases to 66.9. Production of HI is favored to some extent by an increase in temperature, but its dissociation to hydrogen and iodine is favored much more.
The hydrogen iodide-producing reaction is exothermic or heat emitting:
-
- H2(g) + I2(g)
 2HI(g)
2HI(g) - ΔH298 = -10.2 kJ per 2 moles of HI
- H2(g) + I2(g)
(If you check this figure against Appendix 3, remember that this reaction involves gaseous iodine, not solid.) If the external temperature of this reaction is lowered, the equilibrium is shifted in favor of the heat-emitting or forward reaction; conversely, if the temperature is increased, the reverse reaction, producing H2 and I2 is favored. The equilibrium shifts so as to counteract to some extent the effect of adding heat externally (raising the temperature) or removing it (lowering the temperature).
The temperature dependence of the equilibrium point is one example of a more general principle, known as Le Chatelier's principle: If an external stress is applied to a system at chemical equilibrium, then the equilibrium point will change in such a way as to counteract the effects of that stress. If the forward half of an equilibrium reaction is exothermic, then Keq will decrease as the temperature increases; if it is endothermic, Keq will increase. Only for a heat-absorbing reaction can the equilibrium yield of products be improved by increasing the temperature. A good way to remember this is to write the reaction explicitly with a heat term:
-
- H2(g) + I2(g)
 2HI(g) + heat(given off)
2HI(g) + heat(given off)
- H2(g) + I2(g)
Then it is clear that adding heat, just like adding HI, shifts the reaction to the left. (see Figure 4-3.)
Pressure
Le Chatelier's principle is true for other kinds of stress, such as pressure changes. The equilibrium constant, Keq, is not altered by a pressure change at constant temperature. However, the relative amounts of reactants and products will change in a way that can be predicted from Le Chatelier's principle.
The hydrogen- iodine reaction involves an equal number (2) of moles of reactants and product. Therefore, if we double the pressure at constant temperature, the volume of the mixture of gases will be halved. All concentrations in moles liter-1 will be doubled, but their ratio will be the same. In Example 12, doubling the concentrations of the reactants and product does not change the equilibrium constant:
-
- Keq =
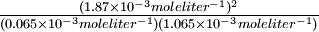
- =
 50.51
50.51
- Keq =
Thus the hydrogen- iodine equilibrium is not sensitive to pressure changes. Notice that in this case Keq does not have units, since the concentration units in the numerator and denominator cancel.
In contrast, the dissociation of ammonia is affected by changes in pressure because the number of moles (2) of reactant does not equal the total number of moles (4) of products:
-
- 2NH3(g)
 N2(g) + 3H2(g)
N2(g) + 3H2(g)
- 2NH3(g)
The equilibrium constant for this reaction at 25°C is
-
- Keq =
![\textstyle\frac{[N_2][H_2]^3}{[NH_3]^2} =](https://upload.wikimedia.org/math/4/e/2/4e2ff16db04c5a821a95e94d95ce0423.png) 2.5
2.5  10-9 mole2 liter -2
10-9 mole2 liter -2
- Keq =
One set of equilibrium conditions is
-
- N2 = 3.28
 10-3 mole liter-1
10-3 mole liter-1 - H2 = 2.05
 10-3 mole liter-1
10-3 mole liter-1 - NH3 = 0.106 mole liter-1
- N2 = 3.28
(Can you verify that these concentrations satisfy the equilibrium condition?) If we now double the pressure at constant temperature, thereby halving the volume and doubling each concentration,
-
- N2 = 6.56
 10-3 mole liter-1
10-3 mole liter-1 - H2 = 4.10
 10-3 mole liter-1
10-3 mole liter-1 - NH3 = 0.212 mole liter-1
- N2 = 6.56
the ratio of products to reactants, the reaction quotient, is no longer equal to Keq:
-
- Q =
 1.0
1.0  10-8 mole2 liter-2
10-8 mole2 liter-2
- Q =

Since Q is greater than Keq, too many product molecules are present for equilibrium. The reverse reaction will run spontaneously, thereby forming more NH3 and decreasing the amounts of H2 and N2. Consequently, part of the increased pressure is offset when the reaction shifts in the direction that lowers the total number of moles of gas present. In general, a reaction that reduces the number of moles of gas will be favored by an increase in pressure, and one that produces more gas will be disfavored. (See Figure 4-4.)
| If the hydrogen iodide reaction were run at a temperature at which the iodine was a solid, would an increase in pressure shift the equilibrium reaction toward more HI, or less? What would be the effect of pressure on Keq? |
|
Solution Since the reaction of 2 moles of gaseous HI now yields 1 mole of gaseous H2 and 1 mole of solid I2 the stress of increased pressure is relieved by dissociating HI to H2 and I2. However, Keq will be unchanged by the pressure increase. |

Catalysis
What effect does a catalyst have on a reaction at equilibrium? None. A catalyst cannot change the value of Keq, but it can increase the speed with which equilibrium is reached. This is the main function of a catalyst. It can take the reaction only to the same equilibrium state that would be reached eventually without the catalyst.
Catalysts are useful, nevertheless. Many desirable reactions, although spontaneous, occur at extremely slow rates under ordinary conditions. In automobile engines, the main smog-producing reaction involving oxides of nitrogen is
-
- N2 + O2
 2NO
2NO
- N2 + O2
(Once NO is present, it reacts readily with more oxygen to make brown N02.) At the high temperature of an automobile engine, Keq for this reaction is so large that appreciable amounts of NO are formed. However, at 25°C, Keq= 10-30. (Using only the previous two bits of information and Le Chatelier's principle, predict whether the reaction as written is endothermic or exothermic. Check your answer using data from Appendix 3.) The amount of NO present in the atmosphere at equilibrium at 25°C should be negligible. NO should decompose spontaneously to N2 and O2 as the exhaust gases cool. But any Southern Californian can verify that this is not what happens. Both NO and N02 are indeed present, because the gases of the atmosphere are not at equilibrium.
The rate of decomposition of NO is extremely slow, although the reaction is spontaneous. One approach to the smog problem has been to search for a catalyst for the reaction
that could be housed in an exhaust system and could break down NO in the exhaust gases as they cool. Finding a catalyst is possible; a practical problem arises from the gradual poisoning of the catalyst by gasoline additives, such as lead compounds. This is the reason why new cars with catalytic converters only use lead-free gasoline.
A proof of the assertion that a catalyst cannot change the equilibrium constant is illustrated in Figure 4-5. If a catalyst could shift the equilibrium point of a reacting gas mixture and produce a volume change, then this expansion and contraction could be harnessed by mechanical means and made to do work. We would have a true perpetual-motion machine that would deliver power without an energy source. From common sense and experience we know this to be impossible. This "common sense" is stated scientifically as the first law of thermodynamics, which will be discussed in Chapter 15. A mathematician would call this a proof by contradiction: If we assume that a catalyst can alter Keq, then we must assume the existence of a perpetual-motion machine. However, a perpetual-motion machine cannot exist; therefore our initial assumption was wrong, and we must conclude that a catalyst cannot alter Keq.
In summary, Keq is a function of temperature, but it is not a function of reactant or product concentrations, total pressure, or the presence or absence of catalysts. The relative amounts of substances at equilibrium can be changed by applying an external stress to the equilibrium mixture of reactants and products, and the change is one that will relieve this stress. This last statement, Le Chatelier's principle, enables us to predict what will happen to a reaction when external factors are changed, without having to make exact calculations.
Summary
A spontaneous reaction is one that will take place, given enough time, without outside assistance. Some spontaneous reactions are rapid, but time is not an element in the definition of spontaneity. A reaction can be almost infinitely slow and still be spontaneous.
The net reaction that we observe is the result of competition between forward and reverse steps. If the forward process is faster, then products accumulate, and we say that the reaction is spontaneous in the forward direction. If the reverse process is faster, then reactants accumulate, and we say that the reverse reaction is the spontaneous one. If both forward and reverse processes take place at the same rate, then no net change is observed in any of the reaction components. This is the condition of chemical equilibrium.
The ratio of products to reactants, each concentration term being raised to a power corresponding to the coefficient of that substance in the balanced chemical equation, is called the equilibrium constant, Keq. (See equation 4-8.) It can be used to predict whether a given reaction under specified conditions will be spontaneous, and to calculate the concentrations of reactants and products at equilibrium. The reaction quotient, Q, has a form that is identical with that of the equilibrium constant, Keq, but Q applies under nonequilibrium conditions as well. For a given set of conditions, if Q is smaller than Keq, the forward reaction is spontaneous; if Q is greater than Keq, the reverse reaction is spontaneous; and if Q = Keq, the system is at equilibrium.
The equilibrium constant can be used with any convenient set of concentration units: moles liter-1 , pressure in atmospheres, or others. Its numerical value will depend on the units of concentration, so one must be careful to match the proper values of Keq and units when solving problems. If gas concentrations are expressed in moles liter-1, the equilibrium constant is designated by Kc; if in atmospheres, by Kp. Just as partial pressure of the jth component of a gas mixture is related to moles per liter by pj = cjRT, so Kp and Kc are related by Kp = Kc(RT)Δn, in which Δn is the net change in number of moles of gas during the reaction.
When some of the reactants or products are pure solids or liquids, they act as infinite reservoirs of material as long as some solid or liquid is left. Their effect on equilibrium depends only on their presence, not on how much of the solid or liquid is present. Their effective concentrations are constant, and can be incorporated into Keq. In practice, this simply means omitting concentration terms for pure solids and liquids from the equilibrium-constant expression. Evaporation of a liquid can be treated formally as a chemical reaction with the liquid as reactant and vapor as product. These conventions for writing concentration terms for a liquid permit us to write the equilibrium constant for evaporation as Kp = pj where pj is the equilibrium vapor pressure of substance j.
Le Chatelier's principle states that if stress is applied to a system at equilibrium the amounts of reactants and products will shift in such a manner as to minimize the stress. This means that for a heat-absorbing, or endothermic, reaction, Keq increases as the temperature is increased, since carrying out more of the reaction is a way of absorbing some of the added heat. Similarly, cooling increases Keq for a heat-emitting or exothermic reaction. Although the equilibrium constant Keq is independent of pressure, and changing the total pressure on a reacting system does not alter Keq directly, an increase in pressure does cause the reaction to shift in the direction that decreases the total number of moles of gas present.
A catalyst has no effect at all on Keq or the conditions of equilibrium. All that a catalyst can do is to make the system reach equilibrium faster than it would have done otherwise. Catalysts can make inherently spontaneous but slow reactions into rapid reactions, but they cannot make nonspontaneous reactions take place of their own accord.


![K_{eq} = \textstyle\frac{[CO_2][H_2]}{[CO][H_2O]}](https://upload.wikimedia.org/math/8/1/c/81c99d309e6f1206b50dc7ff9aa92680.png)
![K_{eq} = \textstyle\frac{[H_2O]^2}{[H_2]^2[O_2]}](https://upload.wikimedia.org/math/2/4/6/246875e68142de3c40832911102d5100.png)
![K_{eq} = \textstyle\frac{[H_2]^2[O_2]}{[H_2O]^2}](https://upload.wikimedia.org/math/f/7/5/f75780e1d3dcee744860ec416c6344b3.png)
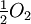
![K_{eq} = \textstyle\frac{[H_2][O_2]^{1/2}}{[H_2O]}](https://upload.wikimedia.org/math/0/9/7/0972b6b068e323e50342085c05a477e8.png)
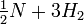
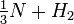
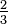 NH3
NH3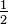 N2 +
N2 + 
![K_{a} = \textstyle\frac{[NH_3]^2}{[N_2][H_2]^3}](https://upload.wikimedia.org/math/a/d/8/ad86d4564aac8b1cda9e05a3eefffcf0.png) c)
c) ![K_{c} = \textstyle\frac{[NH_3]^{2/3}}{[N_2]^{1/3}[H_2]}](https://upload.wikimedia.org/math/f/7/5/f757becfc6583f9321e4106bdfa8cd7b.png)
![K_{b} = \textstyle\frac{[NH_3]^2}{[N_2]^{1/2}[H_2]^{3/2}}](https://upload.wikimedia.org/math/a/8/a/a8a5314f8ca783f5849e6de01daf00b6.png) d)
d) ![K_{d} = \textstyle\frac{[N_2]^{1/2}}{[H_2]^{3/2}[NH_3]}](https://upload.wikimedia.org/math/a/6/6/a66109e186a5e0a05c3f21eeda4ff15a.png)

 = 0.040
= 0.040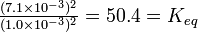
![\textstyle\frac{[HI]^2}{(1.0 \times 10^{-3})^2} = K_{eq}](https://upload.wikimedia.org/math/8/7/2/8727ce0aa7b426542e2508e20e9b7476.png) = 50.53
= 50.53![[HI]^2 = 50.53 \times 1.0 \times 10^{-6}](https://upload.wikimedia.org/math/b/d/1/bd16b32b5b1f6c52889415d385aea896.png)
![\textstyle [HI] = 7.1 \times 10^{-3} \textstyle mole liter^{-1}](https://upload.wikimedia.org/math/2/c/a/2cab478a8df4ae5dc2ef8cf5352a461f.png)
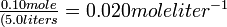

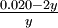
![\textstyle\frac{2y}{[HI]_0} = \frac{0.0044}{0.020}](https://upload.wikimedia.org/math/1/2/c/12cf43f99b7d30ac9751410fed8007cd.png) = 0.22, or 22% dissociation
= 0.22, or 22% dissociation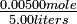 = 0.00100 mole liter-1
= 0.00100 mole liter-1 = 0.00200 mole liter-1
= 0.00200 mole liter-1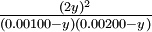

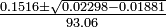
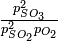 (pj = partial pressure of j)
(pj = partial pressure of j)
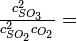 287 moles-1 liter
287 moles-1 liter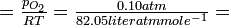 0.00122 mole liter-1
0.00122 mole liter-1
![\textstyle\frac{[HI]^2}{[H_2]}](https://upload.wikimedia.org/math/f/d/f/fdfe56442332ce0e0fc27ca90b0ccd65.png)
![\textstyle\frac{[CO_2]^2}{[CO]^2}](https://upload.wikimedia.org/math/c/3/6/c360a1ad89af9307345447bc04268660.png)
![\textstyle\frac{[CO]}{[CO_2][H_2]}](https://upload.wikimedia.org/math/0/8/b/08b72caa66c89fe78271a5944718837b.png)
![\textstyle\frac{[CO][H_20]}{[CO_2][H_2]}](https://upload.wikimedia.org/math/4/d/3/4d33a02b44cfc295a0b768043e40b8e0.png)