8.12: Ice and Water
- Page ID
- 49459
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The next simplest, and by far the most important, example of hydrogen bonding is that which occurs in H2O. Again there is clear evidence of hydrogen bonding in the structure of the solid. Figure 1 shows two computer-drawn diagrams of the crystal lattice of ice. In the model we can clearly see that each O atom is surrounded by four H atoms arranged tetrahedrally. Two of these are at a distance of 99 pm and are clearly covalently bonded to the O atom. The other two are at a distance of 177 pm.
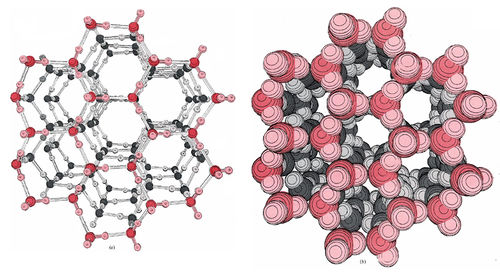
They are covalently bonded to other O atoms but are hydrogen bonded to the one in question. The situation is thus:
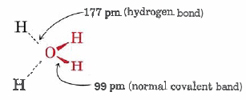
As in the case of HF, the distance between molecules is abnormally short. The sum of the van der Waals radii of H and O is 260 pm, considerably larger than the observed 177 pm.
The tetrahedral orientation of H atoms around O atoms which results from hydrogen-bond formation has a profound effect on the properties of ice and of liquid water. In the space-filling diagram of ice, most of the electron density of each H and O atom is enclosed by a boundary surface. As you can see, hydrogen bonding causes the H2O molecules to adopt a rather open structure with hexagonal channels running through it. These channels contain an almost perfect vacuum-in them there is a little electron density from the surrounding atoms, but nothing else.
When ice melts, some of the hydrogen bonds are broken and the rigid crystal lattice collapses somewhat. The hexagonal channels become partially filled, and the volume of a given amount of H2O decreases. This is the reason that ice is less dense than water and will float on it. As the temperature is raised above 0°C, more hydrogen bonds are broken, more empty space becomes occupied, and the volume continues to decrease. By the time 4°C has been reached, increased molecular velocities allow each H2O molecule to push its neighbors farther away. This counteracts the effect of breaking hydrogen bonds, and the volume of a given amount of H2O begins to increase with temperature.
Most solids expand when they melt, and the corresponding liquids expand continually with increasing temperature, so the behavior of water is rather unusual. It is also extremely important in the environment. When water freezes in small cracks in a rock, the greater volume of the ice can split the rock into smaller pieces. These eventually become able to support plant life, and so water contributes to the formation of fertile soil. The same process happens to roadways, and is the reason for new cracks and potholes seen on roads after a cold winter. The ice bomb experiment, seen below, is perhaps the the most dramatic example of water expanding when frozen.
In the video, water is poured into a cast iron container, which is tightly sealed. The container is then placed in a acetone/dry ice sludge, which is at a temperature of -77°C. After a short period of time, the ice freezes, expands, and causes the cast iron container to explode, blasting off the cover of the acetone/dry ice bath, and spraying the bath itself everywhere. Even though the cast iron container had ⅛ inch thick sides, the pressure of the expanding ice was still able to blow it apart.
Since water has maximum density at 4°C, water at that temperature sinks to the bottom of a deep lake, providing a relatively uniform environment all year around. If ice sank to the bottom, as most freezing liquids would, the surface of a lake would not be insulated from cold winter air. The remaining water would crystallize much more rapidly than it actually does. In a world where ice was denser than water, fish and other aquatic organisms would have to be able to withstand freezing for long periods.
Hydrogen bonding also contributes to the abnormally large quantities of heat that are required to melt, boil, or raise the temperature of a given quantity of water. Heat energy is required to break hydrogen bonds as well as to make water molecules move faster, and so a given quantity of heat raises the temperature of a gram of water less than for almost any other liquid. Even at 100°C there are still a great many unbroken hydrogen bonds, and almost 4 times as much heat is required to vaporize a mole of water than would be expected if there were no hydrogen bonding. This extra-large energy requirement is the reason that water has a higher boiling point than any of the other hydrides.
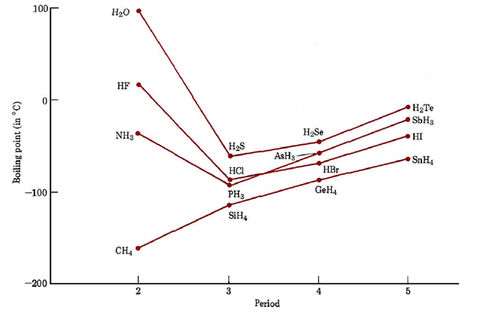
The fact that it takes a lot of heat to melt, boil, or increase the temperature of water, makes this liquid ideal for transferring heat from one place to another. Water is used by engineers in automobile radiators, hot-water heating systems, and solar-energy collectors. More significantly, circulation (in the bloodstream) and evaporation (from the skin) of water regulate the temperature of the human body. (You are between 55 and 65 percent water if female and between 65 and 75 percent water if male.) Because of this (as well as for many other reasons) water is an important component of living systems. Water’s ability to store heat energy is also a major factor affecting world climate. Persons who live near large lakes or oceans experience smaller fluctuations in temperature between winter and summer than those who inhabit places like Siberia, thousands of kilometers from a sizable body of water. Ocean currents, such as the Gulf Stream, convey heat from the tropics to areas which otherwise would be quite cold. It is interesting to ask, for example, whether European civilization could have developed without the aid of warmth transported by the common, but highly unusual, liquid—water.


