2.4: Macroscopic and Microscopic Views of a Chemical Reaction
- Page ID
- 49356
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
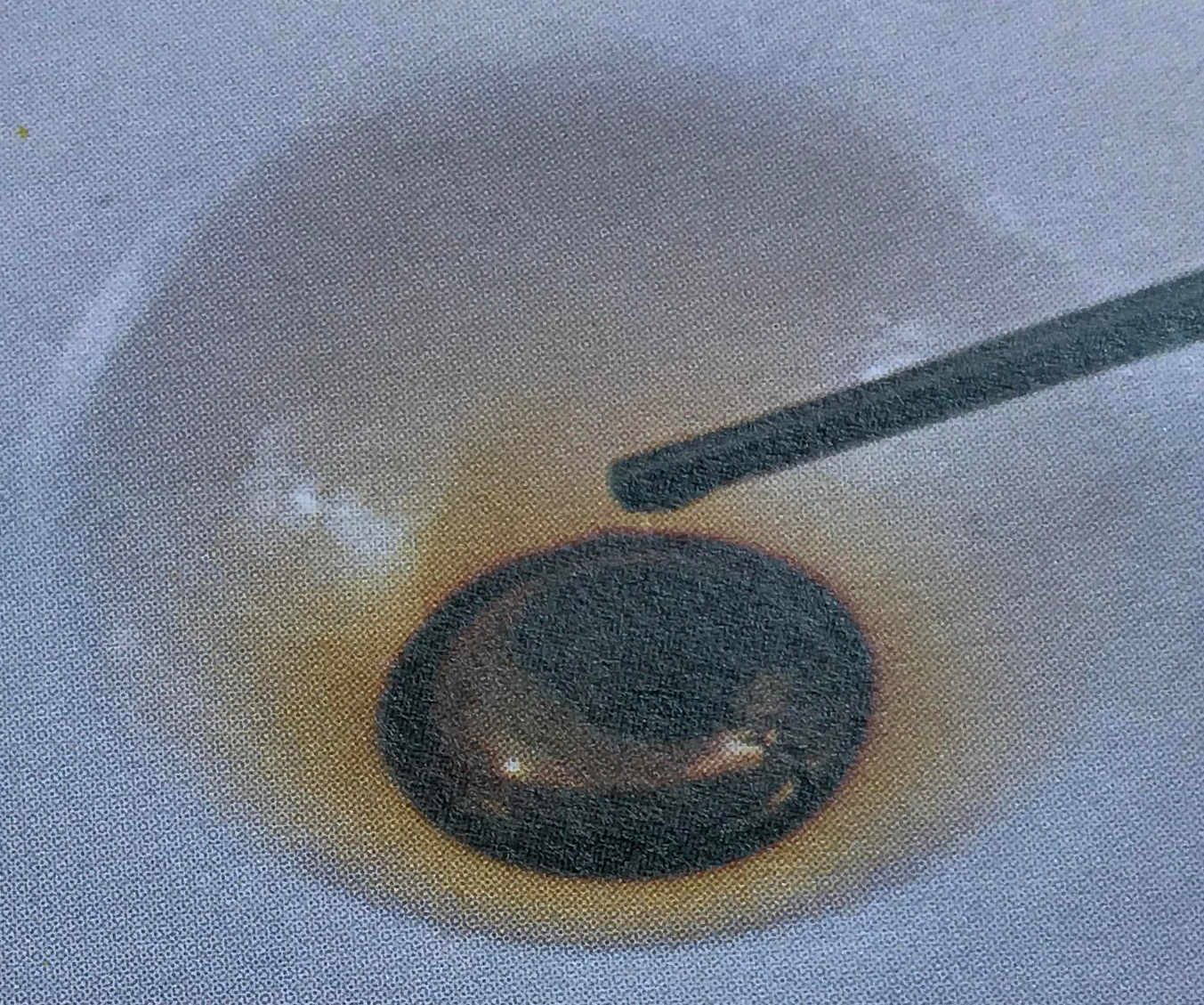
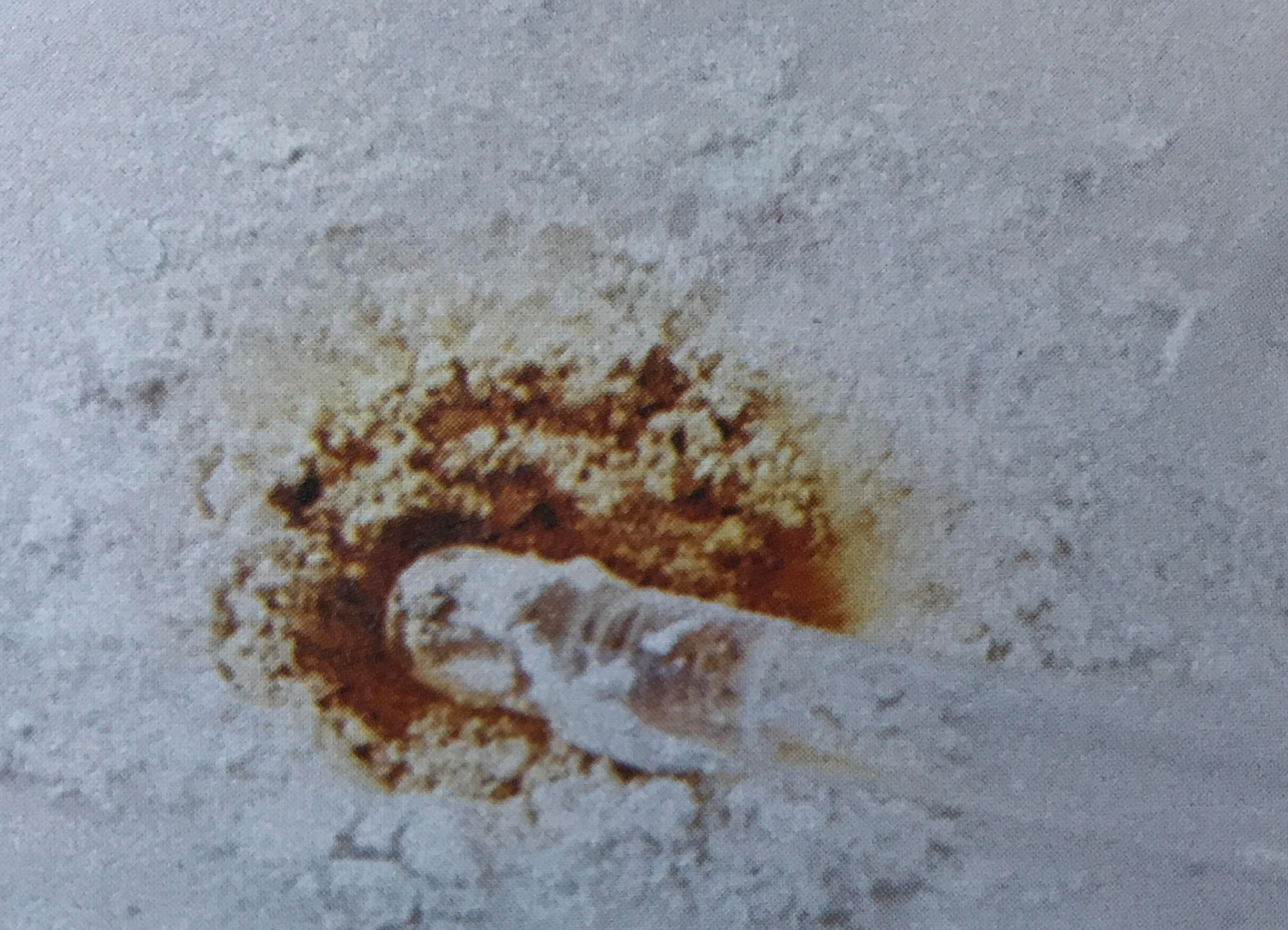
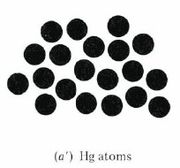
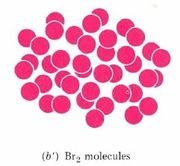
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Dalton’s third postulate(opens in new window) states that atoms are the units of chemical changes. What this means can be seen in the macroscopic and microscopic views of a chemical change in the video and figure farther down this page. The video shows that when macroscopic quantities of mercury and bromine are mixed at room temperature, a chemical reaction occurs, and a new substance, Mercury(II) bromide (mercuric bromide), is produced. Mercury(II) bromide is a white solid, quite different in appearance from the two elements from which it was formed.
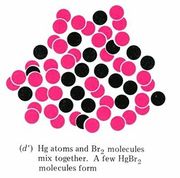
A chemist’s sub-microscopic (nanoscale or atomic scale) theory about what is going on is shown below the video of the macroscopic reaction. Soon after the two liquids are mixed together, a rearrangement of atoms begins. The two bromine atoms of each Br2 molecule become separated and combine instead with mercury atoms. When the chemical reaction is complete, all that remains is a collection of mercury(II) bromide molecules, each of which contains one mercury atom and two bromine atoms. Notice that there are just as many mercury atoms after the reaction as there were before the reaction. The same applies to bromine atoms. Atoms were neither created, destroyed, divided into parts, or changed into other kinds of atoms during the chemical reaction.
The view of solid mercury(II) bromide shown in part f is our first sub-microscopic example of a compound. Each molecule of a compound is made up of two (or more) different kinds of atoms. Since these atoms may be rearranged during a chemical reaction, the molecules may be broken apart and the compound can be decomposed into two (or more) different elements.
The formula for a compound involves at least two chemical symbols—one for each element, present. In the case of mercury(II) bromide each molecule contains one mercury atom and two bromine atoms, and so the formula is HgBr2. Both part f of the figure and the formula tell you that any sample of pure mercury(II) bromide contains twice as many bromine atoms as mercury atoms. This 2:1 ratio agrees with Dalton’s fourth postulate that atoms combine in the ratio of small whole numbers.
Although John Dalton originally used circular symbols like those in the figure to represent atoms in chemical reactions, a modern chemist would use chemical symbols and a chemical equation like:
\[\underset{\text{Reactants}}{\ce{Hg + Br2}} \rightarrow \underset{\text{Products}}{\ce{HgBr2}} \label{1} \]
This equation may be interpreted at the sub-microscopic scale to mean that 1 mercury atom and 1 bromine molecule react to form 1 mercury(II) bromide molecule. It should also call to mind the macroscopic change shown in the video, in which a silvery liquid and a red-brown liquid change into a white solid. This macroscopic interpretation is often strengthened by specifying physical states of the reactants and products:
\[\text{Hg}(l) + \text{Br}_2 (l) \rightarrow \text{HgBr}_2 (s) \label{2} \]
Thus liquid mercury and liquid bromine react to form solid mercuric bromide. [If the bromine had been in gaseous form, Br2(g) might have been written as a product of the reaction. Occasionally (c) may be used instead of (s) to indicate a crystalline solid.] Chemical equations such as \(\ref{1}\) and \(\ref{2}\) summarize a great deal of information on both macroscopic and sub-microscopic levels, for those who know how to interpret them.
 |
 |
 |
 |
 |
_Bromide_Rotated.png?revision=1) |
|---|---|---|---|---|---|
 |
 |
 |
 |

|
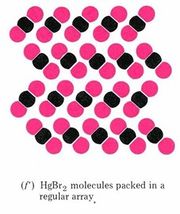 |
Figure \(\PageIndex{1}\) Macroscopic and sub-microscopic descriptions of a chemical reaction. On the macroscopic level a silvery liquid, mercury, is mixed with a red-brown liquid, bromine, and white crystals are produced, as shown in the video above. On the sub-microscopic (nanoscale) level Hg atoms combine with Br2 molecules to form HgBr2 molecules packed together in a regular array. These images are below in sequential order.


