7.7: Solutions for Selected Problems
- Page ID
- 202970
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Exercise 7.2.1:
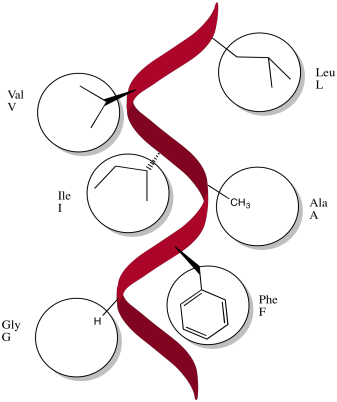
Exercise 7.2.2:
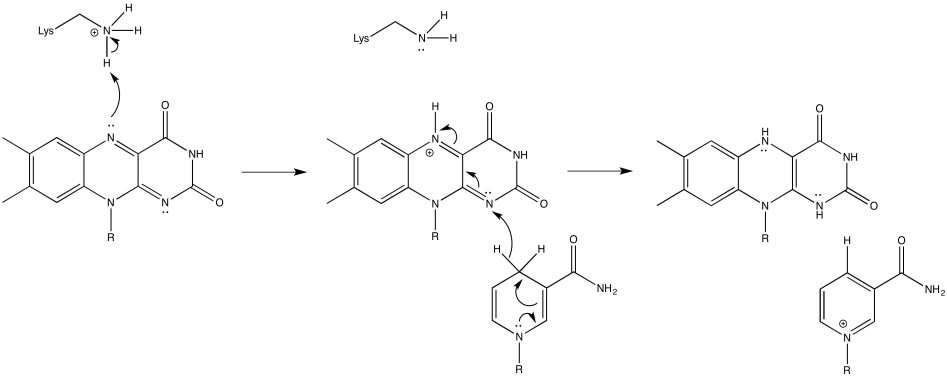
Exercise 6.2.3:
a) Iron charges: \(Fe(II) + Fe(III) = 5^{+}\)
Ligand charges: \(2 \: sulfides \: = 2 \times 2^{-} = 4^{-} ; 4 \: cysteines \: = 4 \times 1^{-} = 4^{-} ; \: total \:= 8^{-}\)
Overall: 3-
b) Iron charges: \(2 \times Fe(II) + Fe(III) = 4^{+} + 3^{+} = 7^{+}\)
Ligand charges: \(4 \: sulfides \: = 4 \times 2^{-} = 8^{-}; 3 \: cysteines \: = 3 \times 1^{-} = 3^{-} ; \: total \: = 11^{-}\)
Overall: 4-
c) Iron charges: \(3 \times Fe(II) + Fe(III) = 6^{+} + 3^{+} = 9^{+}\)
Ligand charges: \(4 \: sulfides \: = 4 \times 2^{-} = 8^{-} ; 4 \: cysteines \: = 4 \times 1^{-} = 4^{-} ; \: total \: = 12^{-}\)
Overall: 3-
Exercise 7.2.4:
Upon reduction, the charge on a 2Fe2S cluster will increase from 3- to 4-, assuming it starts in a mixed Fe(II)/(III) state (whereas if it starts in a Fe(III)/(III) state, the overall charge will increase from 2- to 3-). These anions would be stabilised by strong intermolecular interactions such as ion-dipole forces. Both states (oxidised and reduced) will be stabilised by a polar environment, but the more highly charged reduced state will depend even more strongly on stabilisation by the environment. As a result, we might expect the reduction potential to be lower when surrounded by nonpolar amino acid residues, and higher if surrounded by polar residues.
Exercise 7.2.5:
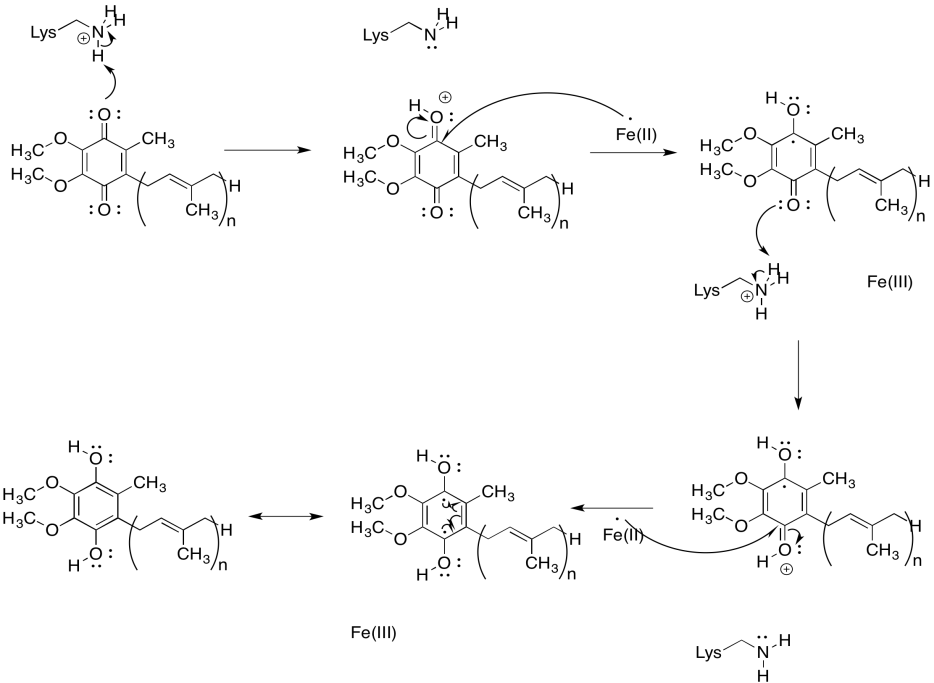
Exercise 7.2.6:

Exercise 7.2.7:
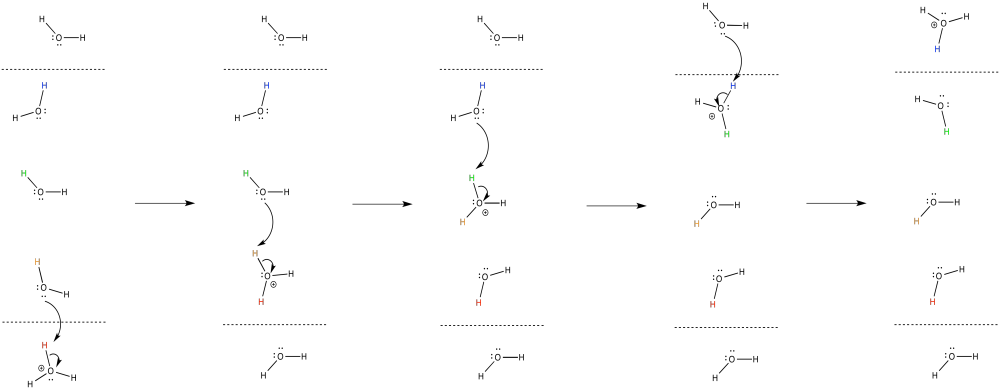
Exercise 7.2.8:
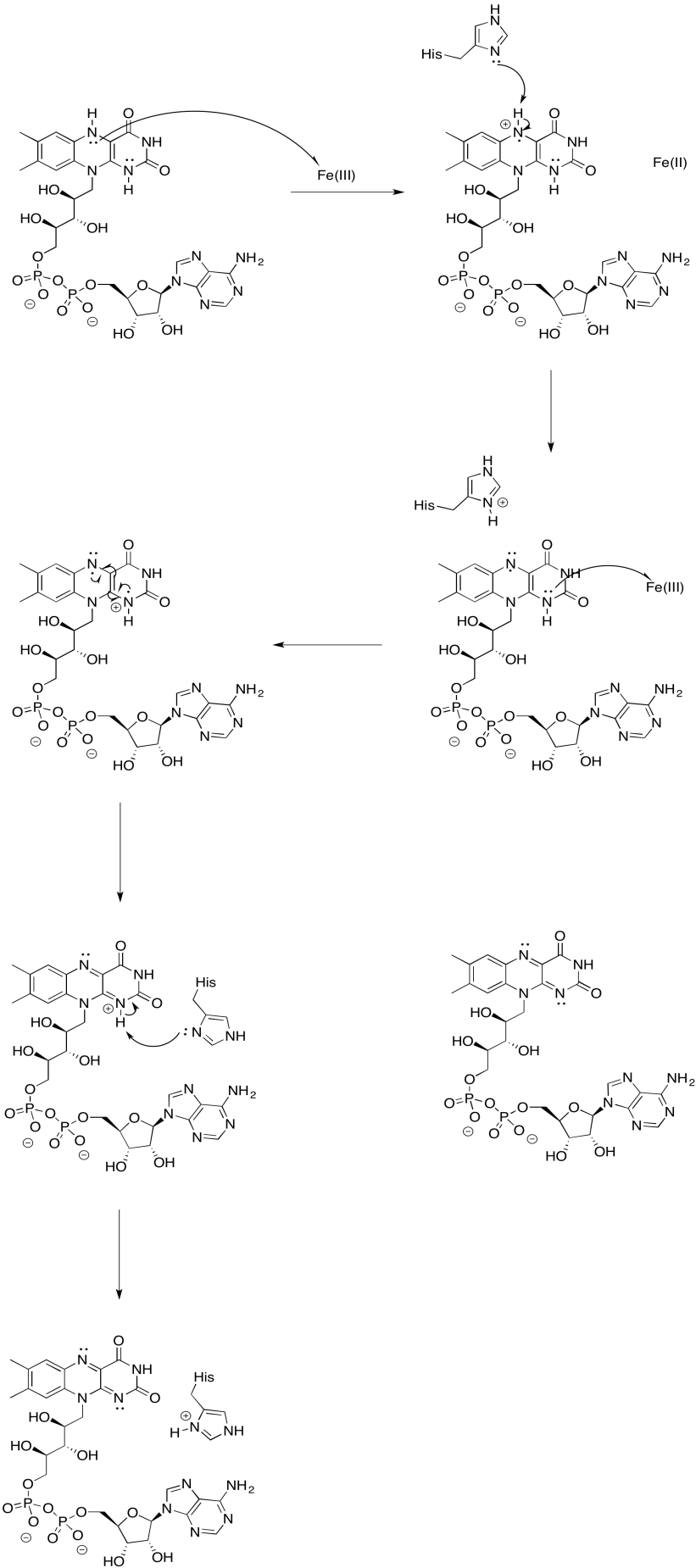
Exercise 7.2.9:
a) N1a and N1b are most likely not involved, because their reduction potentials are too negative.
b)
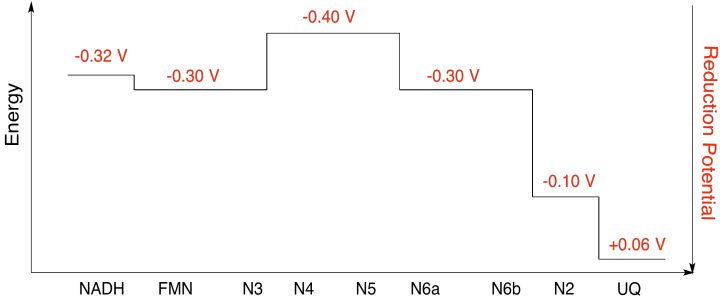
Exercise 7.2.10:
Assuming the reduction potentials are:
\[N5(ox) + e^{-} \rightarrow N5(red)\) \(E^{o}_{red}= -0.40V \nonumber\]
\[N6a(ox) + e^{-} \rightarrow N6a(red)\) \(E^{o}_{red} = -0.30V \nonumber\]
Then the potential difference for the reaction, \(\Delta E^{o} = -0.30 - (-0.40)V = 0.10V\)
The Faraday relation \(\Delta G= -n F \Delta E^{o} \) gives
\[\Delta G = -1 \times 96485 \frac{J}{V \: mol} \times 0.10 V = 9649 \frac{J}{mol} = 9.7 \frac{kJ}{mol} \nonumber\]
Exercise 7.3.1:
Heme b.
Exercise 7.3.2:
A porphyrin contains four pyrrole rings (five-membered, aromatic ring containing a nitrogen) arranged to form a 16-membered macrocycle.
Exercise 7.3.3:
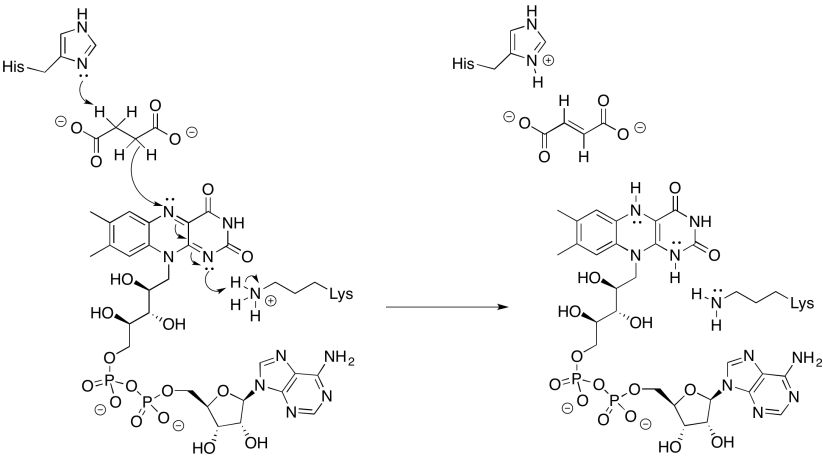
Exercise 7.3.4:
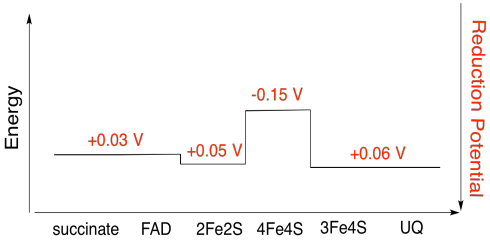
Exercise 7.3.5:
Assuming the reduction potentials are:
\[4Fe4s(ox) + e^{-} \rightarrow 4Fe4S(red)\) \(E^{o}_{red} = -0.15V \nonumber\]
\[3Fe4S(ox) + e^{-} \rightarrow 3Fe4S(red)\) \(E^{o}_{red} = 0.06V \nonumber\]
Then the potential difference for the reaction, \(\Delta E^{o} = 0.06 -(-0.15)V = 0.21V\)
The Faraday relation \(\Delta G = -n F \Dleta E^{o}\) gives
\[\Delta G = -1 \times 96485 \frac{J}{V \: mol} \times 0.21 V = 20262 \frac{J}{mol} = 20 \frac{kJ}{mol} \nonumber\]
Exercise 7.3.6:
a) Iron charges: \(2 \times Fe(III) = 6^{+}\)
Ligand charges: \(2 \: sulfides \: = 2 \times 2^{-}= 4^{-} ; 4 \: cysteines \: = 4 \times 1^{-} = 4^{-} ; \: total \: = 8^{-}\)
Overall: 2-
b) Iron charges: \(3 \times Fe(III) = 9^{+}\)
Ligand charges: \(4 \: sulfides \: 4 \times 2^{-} = 8^{-} ; 3 \: cysteines \: = 3 \times 1^{-} = 3^{-} ; \: total \: = 11^{-}\)
Overall: 2-
c) Iron charges: \(4 \times Fe(III) = 12^{+}\)
Ligand charges: \(4 \: sulfides = 4 \times 2^{-} = 8^{-} ; 4 \: cysteines \: = 4 \times 1^{-} = 4^{-} ; \: total \: = 12^{-}\)
Overall: 0
Exercise 7.4.1:
Arninine and lysine are positively charged at neutral pH.
Exercise 7.4.2:
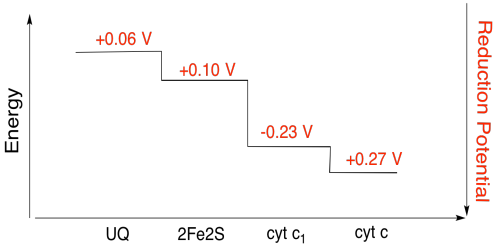
Exercise 7.4.3:
Assuming the reduction potentials are:
\[2Fe2S(ox) + e^{-} \rightarrow 2Fe2S(red)\) \(E^{o}_{red} = 0.10V \nonumber\]
\[cyt \: c_{1} (ox) + 3^{-} \rightarrow cyt \: c_{1} (red)\) \(E^{o}_{red}= 0.230V \nonumber\]
Then the potential difference for the reaction, \(\Delta E^{o} = 0.23- (0.10) V = 0.13V\)
The Faraday relation \(\Delta G = -nF \Delta E^{o}\) gives
\[\Delta G = -1 \times 96485 \frac{J}{V \: mol} \times 0.13V = 12543 \frac{J}{mol} = 12.5 \frac{kJ}{mol} \nonumber\]
Exercise 7.4.4:
The positive arginine residues would confer partial positive charge on the ubiquinone via hydrogen bonding; the ubiquinone would have a more positive reduction potential as a result.

Exercise 7.5.1:
\[\ce{O2 -> H2O} \nonumber\]
\(\ce{O2 -> 2H2O}\) (O balanced)
\(\ce{O2 + 4H^{+} -> 2H2O}\) (H balanced)
\(\ce{O2 + 4e^{-} + 4H^{+} -> 2H2O}\) (charge balanced)
Exercise 7.5.2:
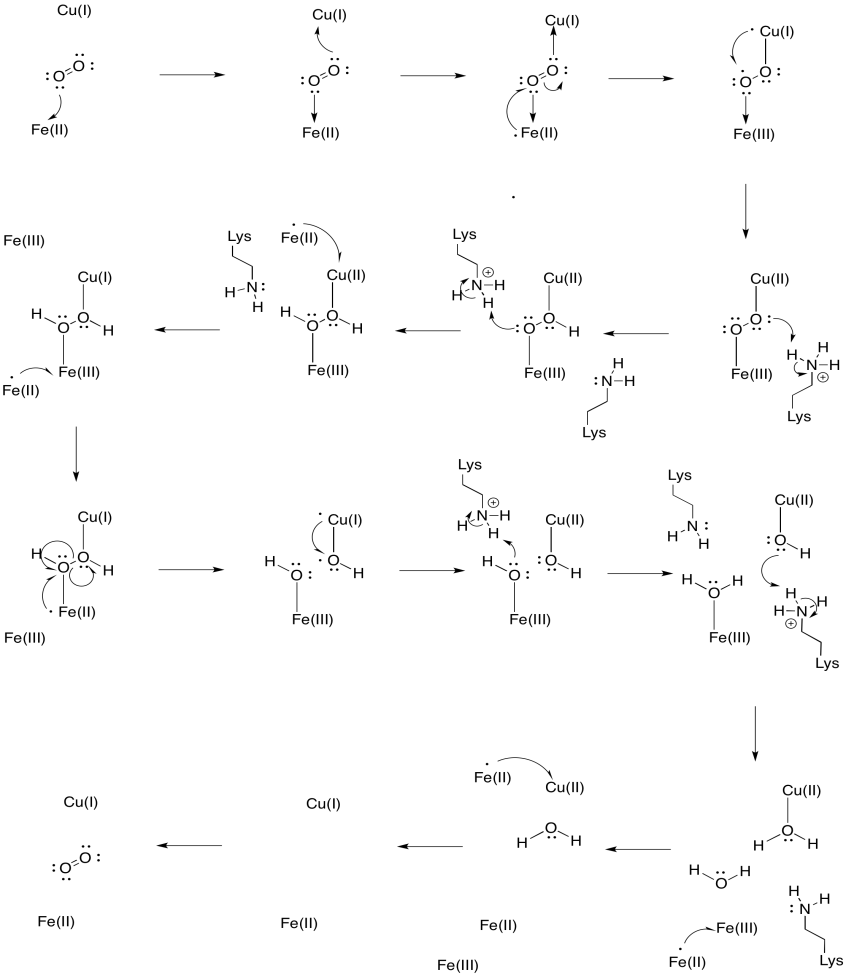
Exercise 7.5.3:
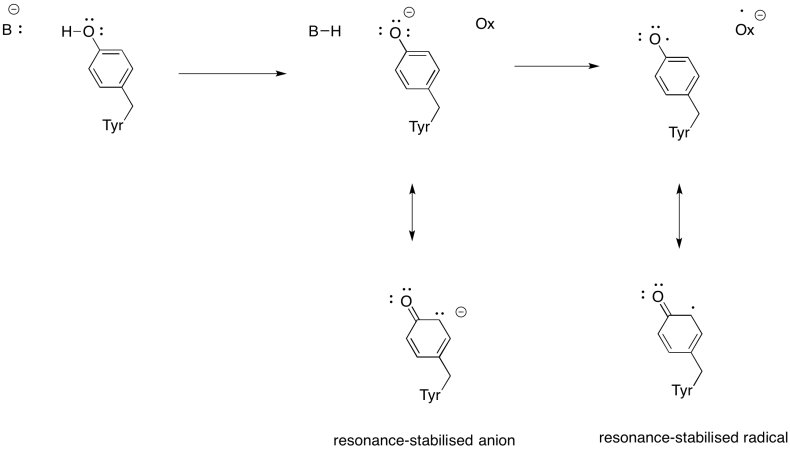
Exercise 7.5.4:
a)
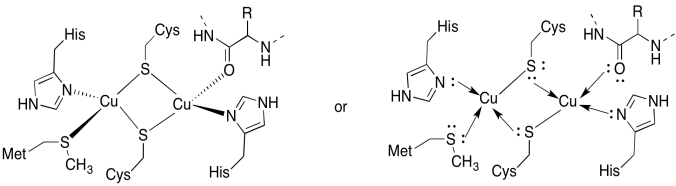
b) tetrahedral
c) Cu(I) = d10
4 donors = 8 e-
total = 18e-
d) 2 x Cu(I) = 2+
2 x Cys-S- = 2-
All others neutral
Total = 0
Exercise 7.5.5:
a)
) trigonal planar
c) Cu(I) = d10
3 donors = 6 e-
total = 16 e-
d) Cu(I) = 1+
histidines neutral
Total = 1+
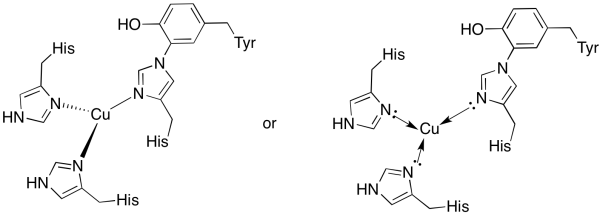
Exercise 7.5.6:
Exercise 7.5.7:
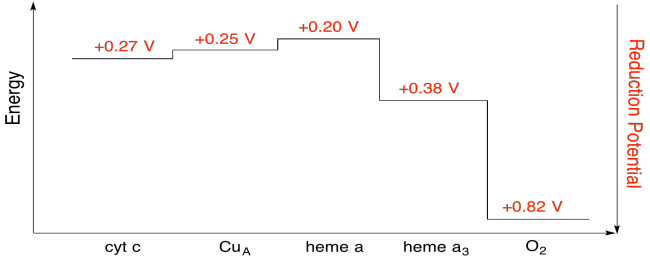
Assuming the reduction potentials are:
\[heme \: a(ox) + e^{-} \rightarrow heme \: a(red)\) \(E^{o}_{red} = 0.20V \nonumber\]
\[heme \: a_{3}(ox) + e^{-} \rightarrow heme \: a_{3}(red)\) \(E^{o}_{red} = 0.38V \nonumber\]
Then the potential difference for the reaction, \(\Delta E^{o} = 0.38 - (0.20)V = 0.18V\)
The Faraday relation \(\Delta G = -n F \Delta E^{o} \) gives
\[\Delta G = -1 \times 96485 \frac{J}{V \: mol} \times 0.13V = 17367 \frac{J}{mol} = 17.4 \frac{kJ}{mol} \nonumber\]
Exercise 7.6.1:
These amino acids would probably be non-polar: alanine, glycine, methionine, isoleucine, leucine, methionine, phenylalanine, tryptophan, valine.
Exercise 7.6.2:
There is always an equilibrium between the protonated state and the deprotonated state in a charged amino acid residue. For this position, an amino acid is needed that is more reliably in the protonated state; that is, the equilibrium lies more heavily to the protonated side of the equation. Because of the resonance-stabilised cation that results from protonation, arginine is much more likely to remain in a protonated state than lysine. That will make for a more efficient millwheel.
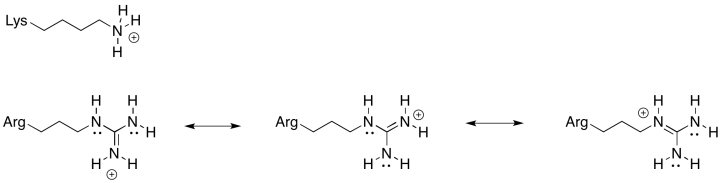
Exercise 7.6.3:
ADP and phosphate are both anions; they would repel normally each other. When bound in the active site, their charges are likely neutralized by complementary charges in the active site.
Exercise 7.6.4:
Assuming the reduction potentials are:
\[\ce{NAD^{+} + 2e^{-} + 2H^{+} -> NADH}\) \(E^{o}_{red} = -0.32V \nonumber\]
\[\ce{0.5 O2 + 2e^{-} + 2H^{+} -> H2O}\) \(E^{o}_{red} = 0.816V \nonumber\]
Then the potential difference for the reaction, \(\Delta E^{o} = 0.816 - (-0.32)V = 1.136V\)
The Faraday relation \(\Delta G = -NF \Delta E^{o}\) gives
\[\Delta G = -2 \times 96475 \frac {J}{V \: mol} \times 1.136V = 219213 \frac{J}{mol} = 219 \frac{kJ}{mol} \nonumber\]
so \(\frac{219 \frac{kJ}{mol}} {30 \frac{kJ}{mol}} = 7.3\)
With 100% efficiency, 7 moles of ATP could be produced per mole of NADH. In reality, about half that amount is produced (closer to 3 moles ATP per mole NADH).


