7.5: Complex IV
- Page ID
- 190049
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Complex IV is the final destination in the electron transport chain. Here, the electrons that have been travelling through the other members of the respiratory supercomplex are finally delivered to O2, reducing it to water. That's an impressive feat, because a number of reactive oxygen species must be formed between the initial addition of an electron and the final release of water, but the reaction is controlled in such a way that the possibility of cell damage is minimised. At the same time, more protons are pumped across the inner mitochondrial membrane.
- In Complex IV, electrons are delivered to their final destination, a molecule of O2.
- The O2 is reduced to water.
Exercise \(\PageIndex{1}\)
Write a balanced redox half-reaction to show how many electrons are needed to reduce an oxygen molecule to water.
- Answer
-
O2 → H2O
O2 → 2 H2O (O balanced)
O2 + 4H+ → 2 H2O (H balanced)
O2 + 4e- + 4H+ → 2 H2O (charge balanced)
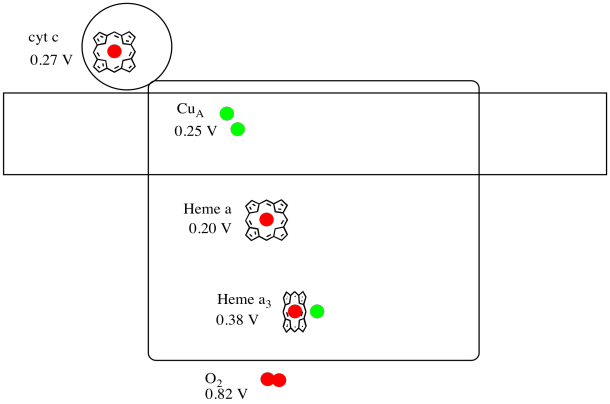
The X-ray structure of Complex IV is shown below. Again, the matrix is at the lower end of the picture and the intermembrane space is at the top. That's where the cytochrome c docks, at the top.
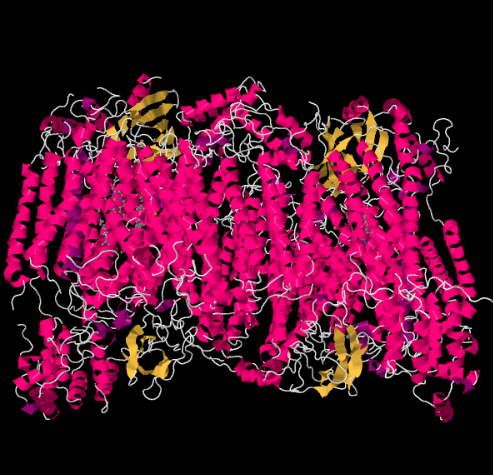
The mobile electron carrier, cytochrome c, binds at Complex IV and delivers an electron to a binuclear copper site called CuA. We can see that binuclear copper site when we look inside the protein, below. It is bound just to the protein and nothing else, so we just see the two copper ions on their own at the top of the picture. This pair of copper atoms sends the electron on to a heme, cytochrome a, which you can see below and to the left. From there, the electron proceeds to another binuclear cluster, this time consisting of a heme-bound iron (cytochrome a3) and a nearby copper (CuB). This binuclear site carries out the reduction of dioxygen to water. In the structure, there is a carbon monoxide molecule bound in the active site between cytochrome a3 and CuB. The carbon atoms is attached to the iron and the oxygen is attached to the copper. That's where the oxygen molecule would bind, waiting to be reduced to water.
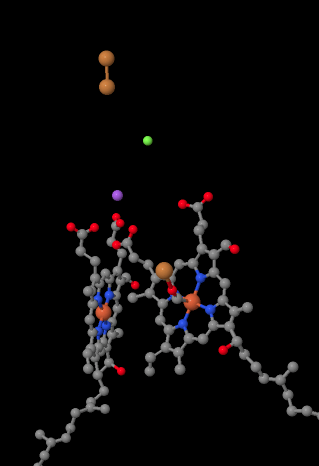
If you think about that, it means that electrons are traveling in the opposite direction from what we saw in the first three complexes. In Complexes I and II, electrons were delivered from the mitochondrial matrix and traveled up toward the intermembrane space, stopping at a ubiquinone in the mitochondrial membrane. In Complex III, electrons continued in that "upward" direction, from the mitochondrial membrane to the cytochrome c in the intermembrane space. In Complex IV, electrons are reversing course, traveling back toward the mitochondrial matrix. Remember, the mitochondrial matrix is n-doped because of proton pumping, so these electrons are traveling from the positive side of the membrane to the negative side. That must be difficult.
- In Complex IV, electron transport is in the opposite direction from the other complexes.
- Electrons travel from the intermembrane space side to the mitochondrial matrix side, against the charge gradient.
Let's take another look at the important ligands for the complex. A cartoon is shown below. In the cartoon, the O2 molecule is shown binding in that position between the heme a3 and the CuB. That dinuclear metal site is where the oxygen molecule is reduced to water.
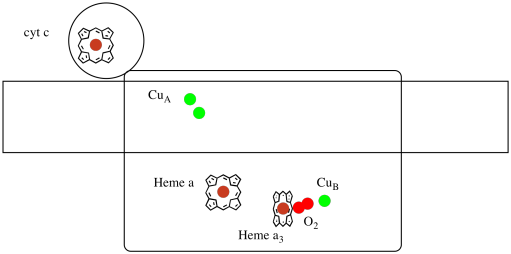
Because four electrons are needed to reduce O2 to H2O, four cytochrome c molecules must bind at Complex IV before that reduction can proceed. It is likely that the coordination environment of the oxygen molecule -- between two metals, rather than just bound to one -- allows it be be more rapidly reduced all the way to water rather than forming reactive oxygen species that persist in the cell, such as peroxides.
Exercise \(\PageIndex{2}\)
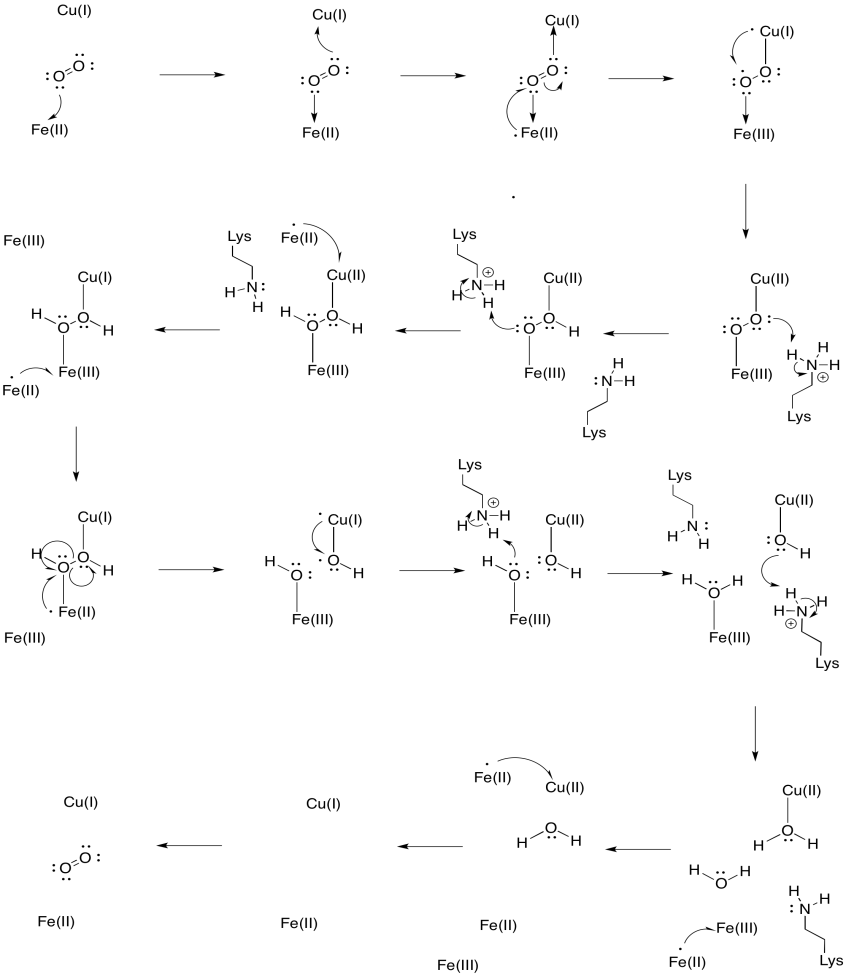
Assume the iron in heme a3 starts in a reduced Fe(II) state and the CuB starts in a reduced Cu(I) state. Provide a mechanism for the reduction of oxygen to water, with the addition of four electrons and four protons. Use Fe(II) as the electron donor and lysine as the proton donor.
- Answer
-
In addition to those two metals, there is also a modified histidine-tyrosine conjugate bound to CuB. It has been suggested that this tyrosine provides another source of immediate electrons that may be used in reduction.
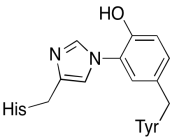
Exercise \(\PageIndex{3}\)
Show why a tyrosine can be a source of both a proton and an electron in biochemical processes.
- Answer
-
In addition to the need to reduce oxygen to water, Complex IV also contributes to the proton gradient, pumping additional protons across the mitochondrial membrane. That task presents additional challenges. A simple coupling mechanism is not possible, because the electrons are moving in the opposite direction. It is though that the mechanism involves conformational changes in the protein that occur as the metals change oxidation states. Subtle changes in coordination environment may result in displacement of amino acid residues nearby. It is easy to imagine that if a particular amino acid shifts upward toward the intermembrane space, it may pull a proton with it.
- Proton pumping and electron transport run in opposite directions in Complex IV and must be uncoupled.
- Proton pumping in Complex IV must rely on conformational changes.
Exercise \(\PageIndex{4}\)
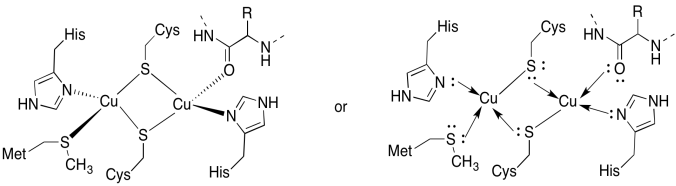
The CuA site contains two coppers, boound by two bridging cysteines. Both are bound by terminal histidines. In addition, one copper is bound by an additional methionine, whereas the other is bound by a carbonyl from the protein backbone.
- Draw the coppers in their binding sites.
- Describe the geometry of each copper.
- If each copper is Cu(I), what is the coordinated electron count on each copper in the complex?
- If each copper is Cu(I), what is the overall charge on the complex?
- Answer a
-
a)
- Answer b
-
b) tetrahedral
- Answer c
-
c) Cu(I) = d10
4 donors = 8 e-
total = 18e-
- Answer d
-
d) 2 x Cu(I) = 2+
2 x Cys-S- = 2-
All others neutral
Total = 0
Exercise \(\PageIndex{5}\)
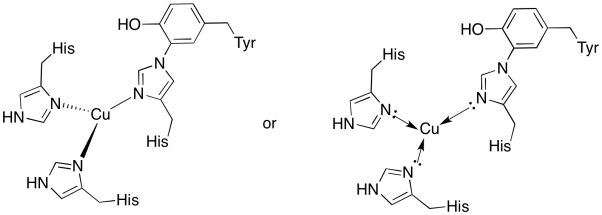
The copper in the CuB site is bound by two histidines and the histidine-tyrosine conjugate.
- Draw the copper in the binding site.
- Describe the geometry of the copper.
- If copper is Cu(I), what is the coordinated electron count in the complex?
- If copper is Cu(I), what is the overall charge on the complex?
- Answer a
-
a)
- Answer b
-
b) trigonal planar
- Answer c
-
c) Cu(I) = d10
3 donors = 6 e-
total = 16 e-
- Answer d
-
d) Cu(I) = 1+
histidines neutral
Total = 1+
Exercise \(\PageIndex{6}\)
It's difficult to measure the reduction potential of an individual site within a protein. However, researchers have been able to estimate these values by measuring EPR spectra under various conditions. Assuming the reduction potentials below, draw a reaction progress diagram for transport of an electron all the way from cytochrome c to molecular oxygen.
- Answer
-
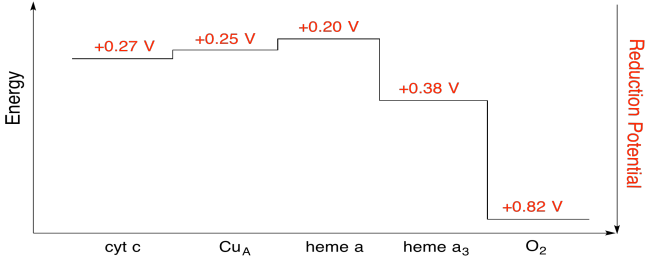
Exercise \(\PageIndex{7}\)
Using the values in the figure above, calculate the energy change when an electron is transferred from heme a to heme a3.
- Answer
-
Assuming the reduction potentials are:
heme a(ox) + e- → heme a(red) Eored = 0.20 V
heme a3(ox) + e- → heme a3(red) Eored = 0.38 V
Then the potential difference for the reaction, ΔEo = 0.38 - (0.20) V = 0.18 V.
The Faraday relation ΔG = - n F ΔEo gives
ΔG = - 1 x 96,485 J V-1 mol-1 x 0.13 V = 17,367 J mol-1 = 17.4 kJ mol-1


