6.5: Radical Propagation
- Page ID
- 190042
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Radicals are known for engaging in "chain reactions". In a chain reaction, a reactive intermediate is generated. When it reacts, it leaves another reactive intermediate, much like the first. This event is called "propagation".
There are a couple of common ways that propagation occurs. The radical might achieve its stable electron count by snatching another atom, especially a hydrogen atom. That event is called hydrogen atom abstraction. Alternatively, a radical may bond with one of the electrons in a pi bond.
In the abstraction of an atom, the radical forms a bond with that atom. That bond gives the radical an even number of electrons again.
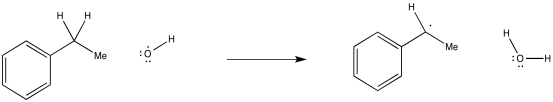
Show, with arrows, the mechanism for abstraction of a hydrogen atom from ethylbenzene by a hydroxyl radical.
- Answer
-
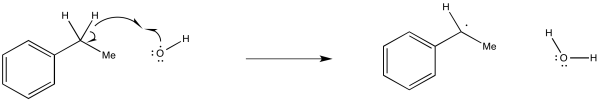
It's important to note that in a hydrogen atom abstraction, the radical is reacting not just with the proton, but with the entire hydrogen atom. It is taking the electron, too.
Exactly which atom gets abstracted has a lot to do with bond strengths. For example, O-H bonds are quite strong (up to 120 kcal/mol, in water, for example). Thus, an OH radical will frequently abstract hydrogen atoms, because there is an energetic payoff when that happens.
Of course, a bond also has to get broken during an abstraction. That costs some energy. C-H bonds are also pretty strong, so they may be hard to break. However, some C-H bonds in particular are weaker than others. For example, in order to break a benzylic C-H bond, the cost is only about 88 kcal/mol. In the case of hydrogen atom abstraction from ethylbenzene by hydroxyl radical, the trade-off is worth it.
Draw a reaction progress diagram for the abstraction of a hydrogen atom from ethylbenzene by a hydroxyl radical.
It is not always the case that a reaction is purely determined by the thermochemistry of the bonds involved. Sometimes, there are kinetic factors that block the path to the more stable product, or that lower the path to the less stable product. However, in many atom abstractions, because the old bond is being broken at the same time that the new bond is being formed, both factors matter in the rate determining step. By the time the transition state is reached, the stability of the complex is influenced both by the bond that is being broken and the bond that is being made. As a result, the thermodynamics of the reaction can have a strong influence on the pathway to products.
| Bond | Dissociation Energy (kcal/mol) | Bond | Dissociation Energy (kcal/mol) |
| F-H | 136 | Br-H | 88 |
| Cl-H | 103 | I-H | 71 |
| EtO-H | 105 | O2N-OMe | 42 |
| CH3S-H | 87 | Cl-OMe | 48 |
| PhO-H | 87 | H3C-OMe | 85 |
| Me2N-H | 91 | H3C-NH2 | 85 |
| Et3Si-H | 96 | H3C-F | 115 |
| Bu3Ge-H | 88 | CH3-H2C-Cl | 85 |
| Bu3Sn-H | 78 | CH3-H2C-Br | 72 |
| Me3Sn-Cl | 100 | CH3-H2C-I | 57 |
Indicate whether a dimethylamine radical is likely to carry out hydrogen atom abstraction from each of the following molecules.
a) Et3SiH b) PhOH c) EtOH d) Bu3SnH e) HF f) HI
- Answer a
-
a) no; a stronger bond would have to be broken and replaced with a weaker bond.
- Answer b
-
b) yes; a weaker bond would be broken and replaced with a stronger one.
- Answer c
-
c) no
- Answer d
-
d) yes
- Answer e
-
e) no
- Answer f
-
f) yes
Indicate whether a chlorine atom abstraction would be likely to occur in each of the following cases.
- Chloroethane is exposed to methoxy radical.
- Chloroethane is exposed to trimethyltin radical.
- Trimethyltin chloride is exposed to methoxy radical.
- Answer a
-
a) no
- Answer b
-
b) yes
- Answer c
-
c) no
Sometimes, the identity of the two atoms that form a bond does not tell the entire story about bond strengths. In the case of C-H bonds of hydrocarbons, for example, a range of bond strengths have been experimentally determined. There is a 40 kcal/mol difference between the weakest C-H bond in a simple hydrocarbon and the strongest (that's about 175 kJ/mol, for the metric-oriented). These bond strengths are remarkably senstive to subtle structural differences, largely because of relative stabilities of the resulting radicals when the bond is broken.
| Bond | Bond dissociation energy (approximate; kcal/mol) |
| HCC-H (aryl) | 130 |
| Ph-H (aryl) | 110 |
| CH2=CH-H (vinyl) | 106 |
| H3C-H | 105 |
| CH3CH2-H | 98 |
| (CH3)2CH-H | 95 |
| (CH3)3C-H | 92 |
| PhCH2-H (benzyl) | 88 |
| CH2=CH-CH2-H (allyl) | 88 |
Propose reasons for the trends in bond strengths among the following groups.
- H3C-H , CH3CH2-H , (CH3)2CH-H, (CH3)3C-H
- (CH3)2CH-H, PhCH2-H, CH2=CH-CH2-H
- HCC-H, CH2=CH-H, CH3CH2-H
- Answer a
-
a) The effect is similar to the stability of carbocations. The more substituted radical is more stable. Thus, the trend from most to least stable is tertiary > secondary > primary > methyl radical. The trend likely originates from a hyperconjugation effect, as in carbocations.
- Answer b
-
b) The trend here is that if the radical is delocalized by resonance, it is more stable. The allyl and benzyl radicals are more stable than the isopropyl radical. This trend is also seen in cations.
- Answer c
-
c) The trend here has to do with "hybridization effects" or the atomic orbitals that contribute to the formation of molecular orbitals involved in the relevant bond. In a linear alkyne, the C-H bond can be formed only from some combination involving a hydrogen 1s orbital, carbon 2s orbital and one of the carbon 2p orbitals. This combination is called a "sp" hybrid and the orbital that combines with the hydrogen can be considered 50% 2s, 50% 2p in character.
In a planar alkene, the C-H bond can be formed only from some combination involving a hydrogen 1s orbital, carbon 2s orbital and two of the carbon 2p orbitals (since two of them could lie in this plane). This combination is called a "sp2" hybrid and the orbital that combines with the hydrogen can be considered 33% 2s, 66% 2p in character.
In a tetrahedral alkane, the C-H bond can be formed from some combination involving a hydrogen 1s orbital, carbon 2s orbital and all three of the carbon 2p orbitals. This combination is called a "sp3" hybrid and the orbital that combines with the hydrogen can be considered 25% 2s, 75% 2p in character.
Because a 2s orbital is lower in energy than a 2p orbital, a bond that has greater 2s character is lower in energy than a bond with less 2s character. That means that a bond with greater 2s character is harder to break than a bond with less 2s character. Hence, the alkane C-H bond is weaker than the alkene C-H bond, which is weaker than the alkyne C-H bond.
Explain why a trialkyltin radical (R3Sn) would not be able to remove a hydrogen atom from propane, but could abstract a chlorine atom from chloroethane.
- Answer
-
The Sn-H bond has a dissociation energy of about 78 kcal/mol, compared to about 98 kcal/mol for the C-H bond in ethane. The formation of the Sn-H bond would not compensate for the energy needed to break the C-H bond. On the other hand, the 100 kcal/mol released upon formation of a Sn-Cl bond would more than make up for the 85 kcal/mol required to breal a C-Cl bond.
We could try to rationalise those differences, although bond strengths are always very complicated issues and we will not be able to explain things satisfactorily without quantum mechanical calculations. Let's start with two basic factors, though: the amount of covalency and the amount of polarity.
The difference between the covalent radii of tin and hydrogen (1.39 vs. 0.31 Å) is much greater than the difference between tin and chlorine (1.39 vs 1.02 Å), so there may be less overlap and less covalency between tin and hydrogen than between tin and chlorine. By comparison, the covalent radius of carbon is about 0.76, which puts it somewhere in between hydrogen and chlorine.
In addition, as measured on the Pauling scale, the electronegativity values of these atoms are: chlorine, 3.16; carbon, 2.55; hydrogen, 2.2; tin, 1.96. The tin-chlorine bond would have a large ionic component; this additional component of bonding would strengthen the Sn-Cl bond.
Addition to an alkene is another common propagation pathway in radical reactions. In this case, a π (pi) bond is broken in the alkene to form a new bond to the radical species. That leaves the second electron from the π bond to form a new radical species.

Π bonds are often weaker than σ bonds, making this pathway energetically accessible in many cases. Perhaps more importantly, the electrons in π bonds are found above and below a flat part of the molecule, leaving them open and accessible for reaction with radicals.
| Bond | Bond dissociation energy (kcal/mol) | Bond | Bond dissociation energy (kcal/mol) |
| H3C-CH3 | 90 | H2C=CH2 | 174 |
| H3C-NH2 | 85 | H2C=NH | ? |
| H3C-OH | 92 | H2C=O | 179 |
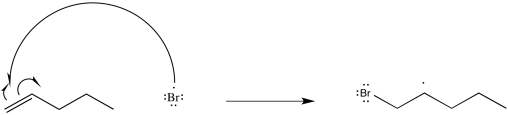
Show a mechanism, with curved arrows, for the reaction of pentene with bromine atom.
- Answer
-
Calculate the strengths of the following pi bonds.
- In ethene.
- In methanal.
- Answer a
-
a) 174 - 90 kcal/mol = 84 kcal/mol for the π contribution only
- Answer b
-
b) 179 - 92 kcal/mol = 87 kcal/mol for the π contribution only
The pi bond in PhCH=NPh has been calculated to have a dissociation energy of 77 kcal/mol.
- Estimate the missing imine C=N bond dissociation energy in the above table.
- Explain why your estimate may be unreliable.
- Answer a
-
a) 85 + 77 kcal/mol = 162 kcal/mol for the combined σ + π contribution
- Answer b
-
b) There may be significant differences between the π bond in methanal imine (CH2=NH) and the imine for which we have bond strength data. For example, breaking the bond would result in radicals next to phenyl groups, which may be significantly stabilized. On the other hand, the π bond itself may be significantly stabilized by conjugation. Thus, our estimate is probably not correct, but it is difficult to say whether it is too high or too low.


