7.3: Distillation
- Page ID
- 189791
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Distillation is a method of purifying organic compounds. It takes advantage of the fact that two different compounds probably have two different boiling points. Suppose two different liquids are present in a homogeneous mixture (they are completely miscible, or they mix completely together, like water and alcohol). If they have two different boiling points, one of the compounds will evaporate before the other one does. The more volatile compound (the one that evaporates easily) will leave the less volatile compound behind.
Distillation is probably a familiar word. Distilleries are factories that produce alcoholic beverages, such as whiskey, from the fermentation of grains, such as corn or rye. At the heart of the distillery is a large distillation apparatus. This "still' is used to purify the alcohol/water mixture by evaporation, leaving behind most of the other components of the grain. That process is slightly more complicated than the one described here, but it is similar.
In distillation, a mixture is put into a container and the container is placed on a heat source. A tube leads away from the container. Sometimes this tube is cooled by cold, running water or ice; sometimes it is just left to cool in the air. There is also a second container to collect the liquid that distills.
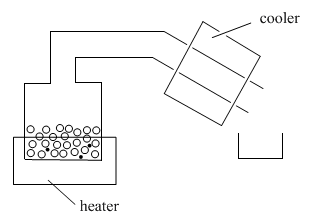
As the container or "pot" is heated, the volatile liquid begins to evaporate. Molecules enter the gas phase and begin to float through the tube leading away from the pot. In the lab, the tube above the pot is called the "still head".

Once molecules get past the still head, they reach the "condensor". Often the condensor is cooled by cold water that flows along outside of it. When molecules reach the cold condenser, they give up some heat energy and condense back into the liquid phase.
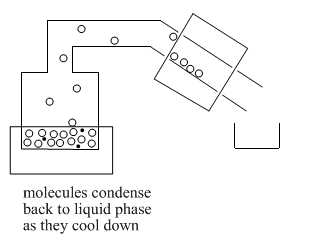
As enough molecules condense into the liquid phase, they begin to form drops of liquid. This liquid collects in a "receiver flask".
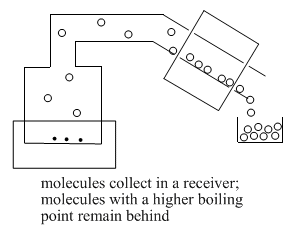
Decide whether the following mixtures are good candidates for distillation.
- You have a sample of pentane (mp: -129°C; bp: 36°C) contaminated with 1-octanol (mp: -16°C; bp: 195°C).
- You have a sample of 1-hexanol (mp: -50°C; bp: 145°C) contaminated with 2-hexanol (mp: -16°C; bp: 140°C).
- You have a sample of benzene (mp: 5°C; bp: 80°C) contaminated with benzoic acid (mp: 122°C; bp: 250°C).
- You have a sample of 2-butanone (mp: -86°C; bp: 80°C) contaminated with toluene (mp: -95°C; bp: 111°C).
- You have a sample of naphthalene (mp: 78°C; bp: 218°C) contaminated with 2-naphthol (mp: 120°C; bp: 285°C).
- You have a sample of 1-hexanol (mp: -50°C; bp: 145°C) contaminated with 1-hexene (mp: -140°C; bp: 63°C).
- Answer
- Answer a
-
Yes, these compounds are both liquids and the boiling point difference is large.
- Answer b
-
No, these compounds are both liquids but the boiling point difference is small.
- Answer c
-
Yes, the compound you want to purify is a liquid and the boiling point difference is large. However, you would have to be very careful not to char the remaining material in the flask.
- Answer d
-
Yes, these compounds are both liquids and the boiling point difference is large.
- Answer e
-
No, these compounds are both solids. Distillation is not a good idea.
- Answer f
-
Yes, these compounds are both liquids and the boiling point difference is large. However, you would have to be careful not to char the desired material; as it distills (after the contaminant is removed), its volume will get smaller. At some point, heat dissipation will become a problem for the remaining material.


