4.2: ¹³C NMR Spectroscopy
- Page ID
- 189769
What Does the Spectrum Look Like?
Since organic compounds are largely based on carbon, 13C NMR spectroscopy is a pretty important tool for studying organic compounds. The 13C isotope is the only isotope of carbon that is "NMR-active"; 12C and 14C atoms do not absorb radio waves in a magnetic field. The 13C NMR spectrum of cyclohexane is shown below.
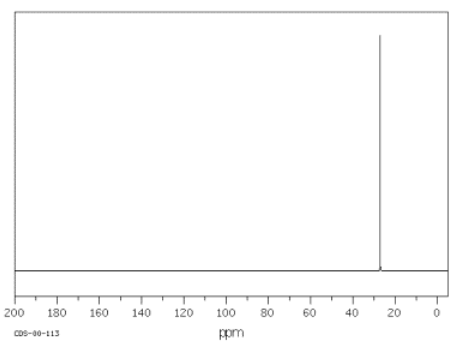
Source: SDBSWeb : http://riodb01.ibase.aist.go.jp/sdbs/ (National Institute of Advanced Industrial Science and Technology of Japan, 15 August 2008)
Cyclohexane is constructed of a ring of six carbon atoms. There are two hydrogen atoms attached to each of the carbons.
Notice these features of the spectrum:
- the x axis corresponds to the energy or frequency of the radio waves that are absorbed.
- the scale on the x axis runs from roughly zero to two hundred "parts per million", abbreviated ppm.
- no scale is given on the y axis, which corresponds to the amount of radio wave "light" absorbed at a given frequency. This situation is different from IR spectroscopy, in which transmittance is plotted rather than absorbance.
- in13C NMR spectroscopy, a peak looks like a spike that rises from the baseline.
There are some peculiar terms used in NMR spectroscopy that are not used in IR spectroscopy. These terms arose from the use of magnetic fields in measuring these spectra:
- the high frequency end of the spectrum, which is high ppm and is found at the left-hand end of the x axis, is termed "downfield".
- the low frequency end of the spectrum, which is low ppm and is found at the right-hand end of the x axis, is termed "upfield".
- the x-axis value is not referred to as "frequency" but rather as "chemical shift" or just "shift".
In cyclohexane, only one frequency of radio waves is absorbed by the carbon atom, and that is at about 27 ppm. Other frequencies could be absorbed by the hydrogen atoms, but hydrogen atoms absorb at very different frequencies from carbon atoms, so they wouldn't be detected in a 13C NMR spectrum.
Which peak would show up farther to the right in the NMR spectrum?
a) 10 pmm or 27 ppm b) 122 ppm or 64 ppm c) 196 ppm or 158 ppm
- Answer
-
a) 10 ppm b) 64 ppm c) 158 ppm
Which peak would show up farther downfield in the NMR spectrum?
a) 17 pmm or 63 ppm b) 201 ppm or 155 ppm c) 71 ppm or 43 ppm
- Answer
-
Which peak would show up farther downfield in the NMR spectrum?
a) 17 pmm or 63 ppm b) 201 ppm or 155 ppm c) 71 ppm or 43 ppm


