5.3: Polar Oxidative Addition
- Page ID
- 189862
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)The polar oxidative addition mechanism is very similar to an aliphatic nucleophilic substitution (SN1 or SN2) reaction.

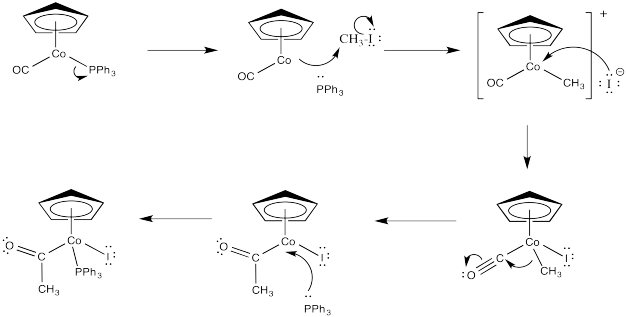
Figure \(\PageIndex{1}\): An example of a polar oxidative addition.
In an oxidative addition, the metal can act as a nucleophile in the first step in an SN2 process. In the second step, the liberated halide binds to the metal. That doesn't happen in a normal nucleophilic substitution. In this case, the metal has donated its electrons and is able to accept another pair from the halide.
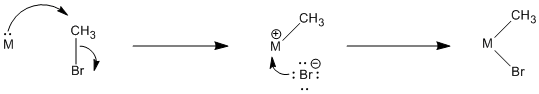
Figure \(\PageIndex{2}\): Mechanistic steps in a polar oxidative addition.
Polar oxidative addition has some requirements similar to a regular SN1 or SN2 reaction:
- Requires good leaving group
- Requires tetrahedral carbon (or a proton) as electrophile
a) What do you think is the most difficult step (i.e. the rate-determining step) for the reaction in Figure \(\PageIndex{2}\) (OA3.2)? Why?
b) Suggest the probable rate law for this reaction.
- Answer a
-
Probably the first step is the hardest (slowest) step, involving bond breaking in the alkyl halide. The donation of the resulting anion to the cation should be pretty fast.
- Answer b
-
\[Rate = k_{1}[ML_{n}][CH_{3}Br] \nonumber\]
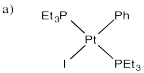
The platinum compound shown below is capable of reductively eliminating a molecule of iodobenzene.

a) Show the products of this reaction.
The starting platinum compound is completely stable in benzene; no reaction occurs in that solvent. However, reductive elimination occurs quickly when the compound is dissolved in methanol instead.
b) Explain why the solvents may play a role in how easily this compound reacts.
The reaction in methanol is inhibited by added iodide salts, such as sodium iodide.
c) Provide a mechanism for the reductive elimination of iodobenzene from the platinum complex, taking into account the solvent dependence and the inhibition by iodide ion.
- Answer a
-
- Answer b
-
Methanol is more polar than benzene. The acceleration of the reaction in methanol suggests that there is increasing polarity in the transition state, or polar intermediates.
- Answer c
-
Inhibition by iodide ion suggests that iodide is a product of a reversible step during this reaction. Adding iodide pushes that step backward, decreasing the rate of product formation. The mechanism below is consistent with these observations:
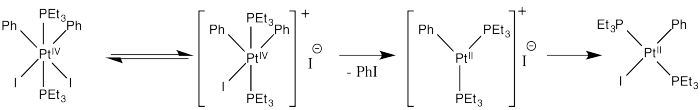
Thus reductive elimination occurs as we go from the second to the third intermediate.
Presumably, the increased positive charge (and general decrease in electron density, owing to loss of a ligand) results in reductive elimination because of destabilization of the Pt(IV).
Alternatively, we might suppose that after loss of iodide, the iodide ion donates directly to a phenyl ligand, displacing the platinum as a leaving group in an SN2 reaction. That would lead directly to the product from the first intermediate, which is a simpler route. However, the precedent for aliphatic nucleophilic substitution involves nucleophilic donation to tetrahedral carbons, not to trigonal planar ones. That mechanism is unlikely.
For the following reaction,
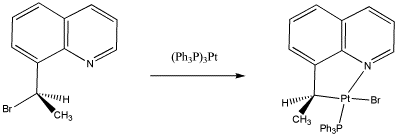
a) Identify the oxidation state at platinum in the reactant and the products.
b) Assign stereochemical configuration in the product and the reactant.
c) Explain the stereochemistry of the reaction.
- Answer a
-
Pt(0) to Pt(II)
- Answer b
-
Changes from (R) to (S)
- Answer c
-
This is an SN2 reaction, so the platinum displaces the bromide from the opposite side.
Reaction of the following deuterium-labeled alkyl chloride with tetrakis(triphenylphosphine) palladium produces an enantiomerically pure product (equation a). Draw the expected product.
However, reaction of a very similar alkyl halide produces a compound that is only 90% enantiomerically pure. Draw the major product and explain the reason that there is some racemization
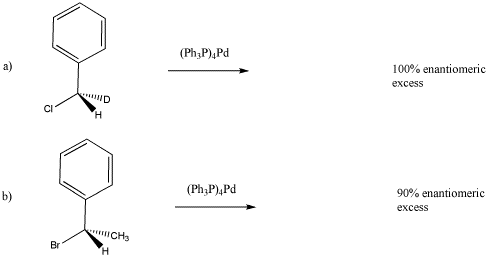
- Answer
-
In case (a), the reaction appears to be SN2, presumably with complete inversion of configuration from (S) to (R). In case (b), there is probably a competing SN1 pathway because the resulting cation is both benzylic and secondary, so it is pretty stable. On the other hand, if the reaction proceeded entirely through an SN1 pathway, the reaction would result in nearly complete racemization, with 0% ee.
Frequently, oxidative additions and reductive eliminations are preceded or followed by other reactions. Draw a mechanism for the following transformation.

- Answer
-