4.12: Elimination
- Page ID
- 200806
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Sometimes, elimination reactions occur instead of aliphatic nucleophilic substitutions. In an elimination reaction, instead of connecting to the electrophilic carbon, the nucleophile takes a proton from the next carbon away from it. The halide or other leaving group is still displaced. A double bond forms between the two carbons.

Thus, there are actually more than two competing mechanisms occurring at once here. In addition to unimolecular and bimolecular substitution, a reaction involving deprotonation is also possible.
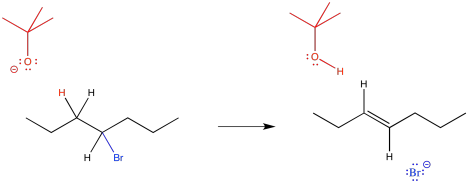
Instead of acting as a nucleophile, the tert-butoxide anion acts as a base. It forms a bond to a proton, becoming tert-butanol. This proton must always come from the carbon next to the leaving group. The bromide still leaves, and the two adjacent carbons form a second bond together.
Draw a mechanism for the elimination reaction above. Assume the reaction is bimolecular and concerted, so that the C-H bond and the C-Br bond break at the same time, forming the C=C bond.
- Answer
-
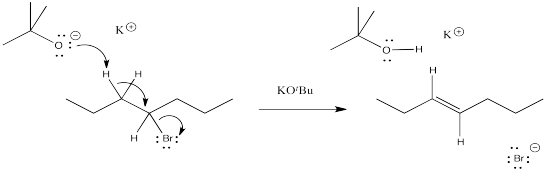
The mechanism of an elimination reaction is almost exactly the same as an aliphatic nucleophilic substitution, except that the nucelophile misses its mark. It hits a proton instead of a carbon and acts as a base instead of a nucleophile. This process can happen at the same time as the leaving group's departure or it can happen afterwards. These mechanisms are called E1 and E2.
Draw another mechanism for the elimination reaction above, but this time, suppose the reaction is unimolecular.
- Answer
-
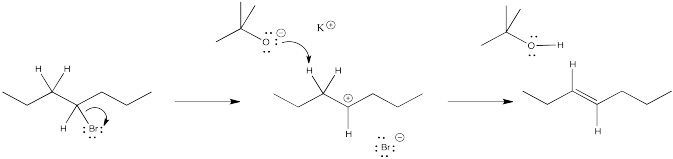
Given the mechanism in Exercise \(\PageIndex{2}\), other products would also be expected.
- What are they? (Think about the reactive intermediate and what else could happen to it.)
- What does their absence in the original reaction scheme above suggest about the most probable mechanism of the reaction?
- Answer a
-
products of cation rearrangement via hydride shifts: 2-heptene instead of 3-heptene.
- Answer b
-
The absence of rearrangement suggests the absence of cations. The mechanism for the reaction shown must be concerted rather than via the ionic intermediate.
Why might a reaction undergo elimination rather than substitution? The most important reason concerns the nature of the nucleophile. The more basic the nucleophile, the more likely it will induce elimination.
- Basic nucleophiles lead to elimination.
What makes something basic, rather than nucleophilic? As a very rough rule of thumb, we can beging by thinking of bases as less stable versions of nucleophiles. Nucleophiles are very often anions, and bases are generally less stable anions. So what factors make anions more stable? Those factors would make the anion more like a nucleophile and less like a base.
One of the most important factors here is polarizability. Remember, polarizable atoms are large atoms. In the main group of the periodic table, they include sulfur, phosphorus, chloride, bromide, iodide, etc. These anions are stable because the negative charge is spread out over a larger atom. Any time charge is spread out, it tends to result in greater stability. On the other hand, smaller, less polarizable atoms include oxygen, nitrogen and carbon. Anions of carbon, nitrogen and oxygen tend to be more basic. Anions of bromine, iodine, and sulfur are not basic. What, never? No, never. Well, hardly ever.
- Large, polarizable atoms such as Br, I, or S make stable anions.
- These stable anions are more likely to be nucleophiles than bases.
You might remember this factor from a discussion of anion stability in acid-base chemistry. Other considerations from acidity will be useful, too.
A second important factor that helped to stabilize anions was resonance. If a negative charge can be delocalised via resonance, the anion becomes much more stable. For example, an alkoxide ion, such as methoxide, might be very basic, because the negative charge is on an oxygen atom. Oxygen is not a large, polarizable atom. However, an adjacent carbonyl in an acetate anion makes all the difference. This anion is resonance stabilised.
- Resonance stabilised anions are relatively stable.
- These stable anions are more likely to be nucleophiles than bases.
Another factor sometimes plays a role in the case of carbon or nitrogen. It is the idea of hybridisation. Remember that the geometry of an atom determines which atomic orbitals are involved in bonding. Tetrahedral carbons are thought of as sp3 hybridised, meaning that the carbon uses an s electron and three p electrons in sigma bonding. A trigonal planar carbon is sp2 hybridised. That means it uses only two p orbitals and an s orbital in forming sigma bonds.
Different hybridisation leads to some subtle differences in properties. For example, the C-H bonds of sp2 carbons are a little stronger than those of sp3 carbons (maybe 105 to 110 kcal/mol for the former, and 95 to 100 kcal/mol for the latter). That's because the electrons in the sp2 C-H bonds are at slightly lower energy. That, in turn, is because an s orbital is a little lower in energy than a p orbital.
For similar reasons, an sp2 carbanion is more stable than an sp3 carbanion. The electrons on the sp2 carbon are lower in energy than the electrons on the sp3 carbon. An sp carbanion is more stable, still. As a result, although an ethyl anion (CH3CH2-) is extremely basic and a vinyl anion (H2C=CH-) is still highly basic, an acetylide or alkynyl anion (HC=C-), though basic, is much more nucleophilic than the other two.
- A sp hybridized carbon anion is much more stable than an sp2 or sp3 carbon anions.
- These relatively stable anions (remember, we are still talking about an anion on carbon), although pretty basic, are usually nucleophiles.
If any of these three factors apply (polarizability, resonance, sp hybridization in carbon), the anion is more likely to be a nucleophile than a base.
There is a fourth factor which is almost a non-sequitur. It goes without saying that a neutral compound -- in the sense that the compound that has no charge at all -- does not require charge stabilisation at all. Thus, if a nucleophile has no charge, it is relatively stable, and will often act as a nucleophile rather than a base.
- Neutral (uncharged) compounds are stable; they do not require charge stabilisation.
- Neutral compounds are usually nucelophiles rather than bases.
A fifth factor -- relative electronegativity within a row -- does play a minor role here. Remember, within a row of the periodic table, size changes are minor. All of the atoms are small. Differences in polarizability are not much of a factor. Instead, differences in electronegativity influence anion stability. The more electronegative the atom, the more stable the anion. As a result, an oxygen anion is more stable than a nitrogen anion or carbon anion. As a result, we sometimes make a distinction here, referring to hydroxide ion and alkoxide ions as strong bases, but amide anions and alkyl anions as very strong bases.
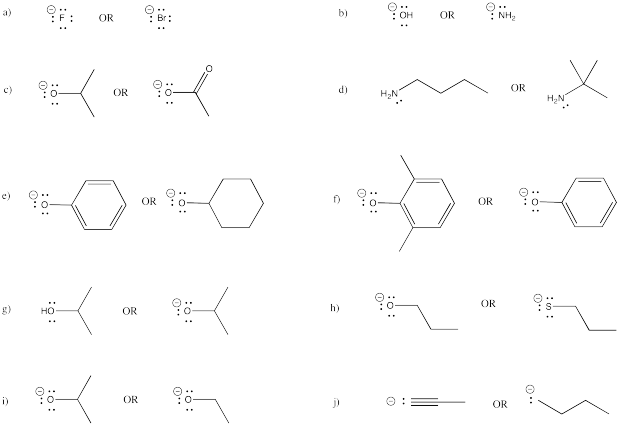
Classify the following anions as very strong base, strong base, or weak base.
a) NaNH2 b) NaOH c) CH3CO2Na d) NaH e) CH3OH
f) NaBr g) CH3COCH2Li h) CH3CH2CH2CH2Li i) KI j) H2O
k) CH3CCNa l) (CH3CH2)3N m) Na2CO3 n) LiCl o) CH3OK
p) CH3C6H4ONa q) NaSH r) [(CH3)2CH]2NLi s) (CH3)3P t) (CH3)3COK
- Answer a
-
very strong
- Answer b
-
strong
- Answer c
-
weak (resonance)
- Answer d
-
very strong
- Answer e
-
weak (neutral)
- Answer f
-
weak (polarizable)
- Answer g
-
weak (resonance)
- Answer h
-
very strong
- Answer i
-
weak (polarizable)
- Answer j
-
weak (neutral)
- Answer k
-
medium-weak (C anion but sp)
- Answer l
-
weak (neutral)
- Answer m
-
weak (resonance)
- Answer n
-
weak (polarizable)
- Answer o
-
strong
- Answer p
-
weak (O anion but delocalised)
- Answer q
-
weak (polarizable)
- Answer r
-
very strong
- Answer s
-
weak (polarizable)
- Answer t
-
strong
Very strong bases include carbon and nitrogen anions and semi-anions. Examples include butyllithium and sodium amide. Very strong bases are highly likely to engage in elimination, rather than substitution.
Strong bases include non-stabilized oxygen anions. Examples include sodium hydroxide as well as alkoxides such as potassium tert-butoxide or sodium ethoxide. Strong bases favor elimination, too. Nevertheless, they can sometimes undergo either elimination or substitution, depending on other factors (see below).
Weak bases include cyanide, stabilized oxygen anions such as carboxylates and aryloxides, sulfur anions, fluoride ion and neutral amines. Weak bases are much more likely to undergo substitution than elimination.

Very weak bases include heavy halides such as chloride, bromide or iodide, as well as neutral phosphorus or sulfur nucleophiles. Very weak bases undergo elimination only rarely.
Typically, strong bases and very strong bases are more likely to react via the E2 mechanism; they react so quickly that the deprotonation step triggers C-LGp ionization, rather than the other way around. However, E1 mechanisms also occur with these bases, especially at low concentrations. Explain why.
Why is it that an anion such as cyanide is a weak base, whereas CH3Li is a strong base?
Another factor is sterics. The more crowded the electrophile, the more likely the nucleophile will encounter a proton on its way to the electrophilic carbon.
As a nucleophile approaches tert-butyl bromide, coming from the side opposite the bromine in order to undergo nucleophilic substitution, it is pretty likely to collide with a proton on its way to the electrophilic carbon. The same thing has a good chance of happening with iso-propyl bromide. However, it is much less likely to happen with bromoethane. Finally, bromomethane doesn't even have a beta-hydrogen, so the chance of elimination in that case is zero.
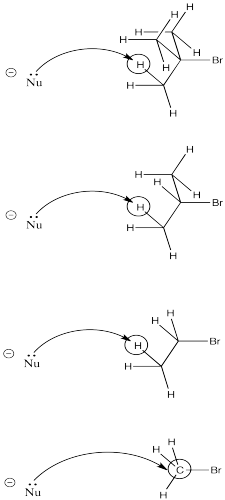
- Crowding leads to elimination.
Note that the crowding could involve either the structure of the base or the structure of the electrophile. A large, bulky base may be more likely to deprotonate than find its way in to the electrophilic carbon atom.
- Bulkier nucleophiles can act as bases.
Given the following pairs of nucleophiles, which one is more likely to undergo elimination?
Although acetylides (such as sodium acetylide, Na CCH) are actually more basic than alkoxides (such as sodium isopropoxide, Na OCH(CH3)2), acetylides frequently undergo substitution rather than elimination. Propose a reason for this difference.
A third factor is temperature. An elimination reaction involves the cleavage of two bonds, whereas a substitution reaction requires only one bond to break. Thus, an elimination reaction is more energy-intensive, and it is more likely to occur at higher temperatures, when more energy is available.
- Higher temperatures lead to elimination.
An additional factor in the energy dependence of eliminations and substitutions is entropy.
- Use simple rules about to determine which products are favored by entropy: Elimination or substitution?
- Given the relationship ΔG = ΔH - T ΔS, which thermodynamic factor dominates free energy change at high temperature?
- Therefore, which product is favored at high temperature?


