4.2: Mechanism of Aliphatic Nucleophilic Substitution
- Page ID
- 189854
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Aliphatic nucleophilic substitution clearly involves the donation of a lone pair from the nucleophile to the tetrahedral, electrophilic carbon bonded to a halogen. We might expect this carbon to be electrophilic because of the halogen attached to it. For that reason, it attracts to nucleophile. However, the mechanism of the reaction might happen in a couple of different ways.
Compare the electronegativity of carbon to that of fluorine, chlorine, bromine and iodine.
- On this basis alone, explain why the carbon attached to the halogen would be electrophilic.
- Which compound should be most electrophilic based on electronegativity: fluoromethane, chloromethane, bromomethane or iodomethane?
- Use the following bond strengths to estimate the qualitative trend in activation barriers for nucleophilic substitution in the four compounds in part (b): C-F 115 kcal/mol; C-Cl 84 kcal/mol; C-Br 72 kcal/mol; C-I 58 kcal/mol.
- Fluorocarbons are quite stable towards aliphatic nucleophilic substitution; in general, they do not undergo this reaction. Explain why.
- Answer a
-
The electronegativity of carbon (2.55 on Pauling scale) is less than that of fluorine (3.98), chlorine (3.16), bromine (2.96) or iodine (2.66).
On that basis, the carbon attached to a halogen is electrophilic because it has a partial positive charge resulting from the polar carbon-halogen bond.
- Answer b
-
We would expect an alkyl fluoride to be the most electrophilic of these compounds, based on electronegativity.
- Answer c
-
Assuming the energy required for breaking the carbon-halogen bond plays a major role in the activation barrier (not guaranteed), we would expect the activation barrier to be lowest with the alkyl iodide, then the alkyl bromide, then the alkyl chloride and finally the alkyl fluoride. This prediction contrasts with what we might expect based on electronegativity.
- Answer d
-
The stability of alkyl fluorides towards this reactions suggests that there is, in fact, a prominent role played by bond strengths, at least in that case. The carbon-fluoride bond is strong enough to hinder nucleophilic substitution in this compound.
In considering possible mechanisms for this reaction, we ought to think about overall bond-making and bond-breaking steps. In the addition of sodium cyanide to alkyl chloride to make an alkyl nitrile, there is one bond-making step (the C-C bond) and one bond-breaking step (the C-Cl bond). The simplest reaction mechanism would involve some combination of these steps.

Two possibilities immediately present themselves:
Mechanism A
The C-C bond forms and then the C-Cl bond breaks.
Mechanism B
The C-Cl bond breaks and then the C-C bond forms.
However, some familiarity with bonding in the second row of the periodic table may suggest to you that mechanism A is not very likely. That mechanism would require forming five bonds to carbon before the C-Cl bond eventually breaks. We can safely ignore this possibility.
Instead, there may be a third possibility to consider.
Mechanism C
The C-Cl bond breaks and the C-C bond forms at the same time.
Mechanism C is a concerted mechanism; two bond-making and -breaking events happen at once. However, no octet rules are violated.
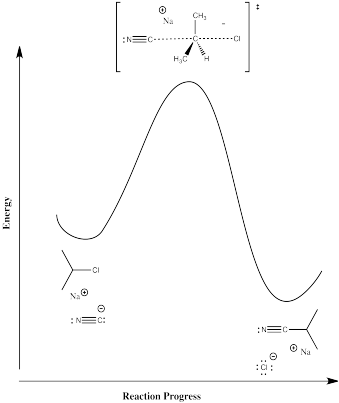
Reaction progress diagrams for these two reactions would look like the illustrations below.
Mechanism B, ionization and then addition of nucleophile:
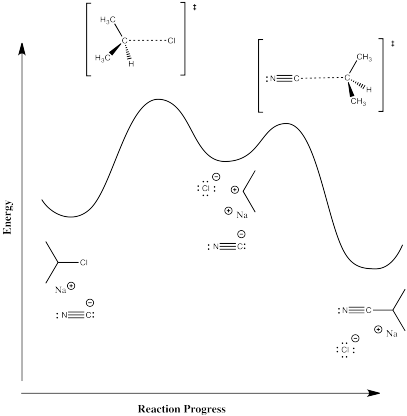
Mechanism C, direct displacement of leaving group by nucelophile:
Compare mechanism B and C in terms of your expectations of the following parameters:
- Activation enthalpy.
- Activation entropy.
- Answer a
-
In mechanism B, the dissociative one, we would expect a higher activation enthalpy. The first step, which appears to be rate determining, is a bond-breaking step, which will cost energy. In mechanism C, the bond-breaking is compensated by some bond-making; overall, this probably costs less energy.
- Answer b
-
In mechanism B, the dissociative case, we expect a more positive entropy of activation. As the bond to the halide begins to break, the halide and carbocation fragments begin to move independently of each other, gaining degrees of freedom and increasing in entropy. In mechanism C, the incoming nucleophile appears to coordinate its motion with that of the departing halide; as a result, there are fewer degrees of freedom in this case.
There isn't necessarily a reason to believe that mechanism B is the correct mechanism and mechanism C is the wrong one, or vice versa. Either one may be possible. You may need to do some work in order to figure out which one really happens. Some experiments may help to highlight what is going on.
If charged intermediates are suspected along a reaction pathway, insight can sometimes be gained by running a reaction in a more polar solvent and comparing its rate to that of the reaction in a less polar solvent.
- Are charged intermediates present, either in mechanism B or C?
- Explain how each of these mechanisms might behave in a more polar solvent.
- Answer a
-
Charged intermediates are present in the dissociative mechanism (B).
- Answer b
-
It seems like a more polar solvent would favor both mechanisms, because both involve the interaction of an anionic nucleophile with an electrophile and loss of an anionic leaving group. However, the dissociative case (B) involves a build-up of charge in the intermediate. It is possible that a more epolar solvent could reduce the barrier to that buildup of charge separation, accelerating this mechanism.
Sometimes, a distinction between two possible mechanisms can be gained by comparing rate laws expected from each mechanism.
- What do you think is the likely rate-determining step in mechanism B?
- What do you expect will be the rate law for mechanism B?
- What do you think is the likely rate-determining step in mechanism C?
- What do you expect will be the rate law for mechanism C?
- Answer a
-
The rate-determining step is probably the bond-breaking one (the first one).
- Answer b
-
Because the nucleophile has not yet participated at that point, \(Rate = k[R-X]\), if R-X = the alkyl halide.
- Answer c
-
There is only one step; it is the rate-determining step, by default.
- Answer d
-
\(Rate = k[R-X][Nu]\).


