8.1: Introduction to Glycolysis - Energy Storage
- Page ID
- 189967
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Glycolysis is a biochemical pathway in which glucose is consumed and ATP is produced. This pathway is an example of catabolism, in which larger molecules are broken down in the cell to make smaller ones. The opposite kind of pathway is anabolism, in which larger molecules are synthesized from smaller ones in the cell.
From the biologist's perspective, catabolism is associated with the breakdown of larger molecules to release energy. For example, in grade school science, you may have learned that most organisms derive their energy from the breakdown of carbohydrates. You may have seen the process of respiration expressed through the following equation of reaction:
\[\ce{C6H12O6_{(s)} + 6O2_{(g)} -> 6CO2_{(g)} + 6H2O_{(l)} + energy} \nonumber\]
That idea gives rise to the slightly misleading paradigm that energy is stored in chemical bonds. The idea goes that, for example, when the single sugar molecule represented by the formula, C6H12O6 , is broken down to make six carbon dioxide molecules, the energy from all of those broken bonds is released for the benefit of the organism.
You may also have learned about another important energy-storage molecule, ATP. Like the breakdown of sugar, the breakdown of ATP is used to power other processes in the cell. That process might be expressed in the following expression:
\[\ce{ATP_{(aq)} + H2O_{(l)} -> ADP_{(aq)} + P_{i(aq)} + energy} \nonumber\]
Once again, this can be considered a breaking-down process, in which an ATP molecule is split into a smaller ADP molecule and an inorganic phosphate.
From the chemist's perspective, it is wrong to suggest that energy is stored in chemical bonds. Instead, energy is released when bonds are formed. This chemical perspective is more than an idea; it represents physical reality. It can be demonstrated in a number of ways that energy is released when bonds are made, and energy must be used up in order to break bonds; apparently, this situation is the opposite of the biological viewpoint.
Some authors have suggested that this apparent disagreement is something like a difference of perspective. Think of an observer standing on the shore of the ocean, watching a ship sail away. From the observer's viewpoint, the ship eventually sinks below the ocean. After a while its hull is no longer visible; only its masts remain, and finally they, too, slip down and are gone. To a passenger on the ship, however, the ship is still sailing along on the surface of the ocean. Biologists and chemists think about bonding differently because they are looking at it from a different viewpoint.
Biologists say that energy is stored in chemical bonds because thinking about things that way is useful to them. It is useful to think of catabolic processes, such as the breakdown of sugars, as energy-releasing. It is useful to think of anabolic processes, such as photosynthesis or the synthesis of complex natural products, as energy-intensive.
Biologists are looking at things purely from the point of view of the biomolecule. Either it is breaking down into smaller pieces (its bonds are breaking), releasing energy, or else it is getting built up into something bigger (its bonds are being made), costing energy.
In a very loose sense, it is as if the reaction of carbohydrate breakdown is pared down to:
\[\ce{C6H12O6_{(s)} -> 6CO2_{(g)} + energy} \nonumber\]
And the reaction of ATP breakdown is abbreviated to:
\[\ce{ATP_{(aq)} -> ADP_{(aq)} + P_{i(aq)} + energy} \nonumber\]
In other words, part of the reaction is ignored. That viewpoint allows a focus on the biomolecule, but it neglects some important things. For example, in the breakdown of carbohydrates, it isn't the C-C bond breaking of the carbohydrates that is the source of energy. It is the formation of strong, new O-H and C=O bonds, and other, more subtle changes, that release the energy.
As always, we get more insight into a reaction by looking at the structural formulae in the equation, rather than condensed formulae. This way, we can actually see what bonds are being made and broken.
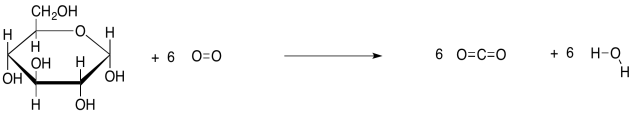
The case of ATP is a little different. The bonds made and broken are pretty much the same in the breakdown of ATP; loosely, we just trade in one P-O bond for another. This case is more complicated, but the simplest explanation is that ATP cleavage relieves repulsion between the multiple negative charges in the ATP molecule. Energy decreases in the resulting molecules, and the rest of the energy that used to be in the reactants is released.
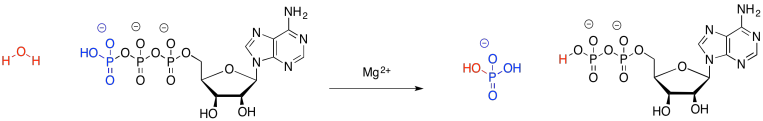
In the reverse, when ADP is phosphorylated to make ATP, the system goes up in energy (the system just means everything in the reaction; it is everything on one side of the arrow or the other). That energy, however, is not really stored in any chemical bonds. It is distributed throughout the system, for example, in the motions of all of those atoms. The bonds may stretch, getting longer and shorter, but in addition the groups on the ends of the bonds can spin, and the molecules can tumble and zip around through space. There are lots of ways to distribute that energy throughout that entire collection of atoms; it isn't forced to sit in that one bond that was newly formed between two atoms.
So, although the idea of energy being stored in chemical bonds may be very useful in the biology classroom, it is only going to get in your way in the chemistry classroom. You need to be able to take off your biologist's hat and put on your chemist's lab coat when you need it.
Our economy is driven largely by the consumption of fossil fuels, such as heptane. Given the following reaction for the breakdown of heptane:
CH3CH2CH2CH2CH2CH2CH3 + 11 O2 → 7 CO2 + 8 H2O
Use the table of bond strengths to determine how much energy is released when a mol of heptane is consumed.
| Bond | O=O | C-C | C-H | C=O | O-H |
| Average Bond Strength (kcal/mol) | 120 | 80 | 100 | 190 | 110 |
- Start by determining the energy needed to break bonds.
- Determine the energy released when new bonds are made.
- Determine the overall energy change.
- Answer
-
Bonds Broken:
C-C 6 x 80 kcal/mol = 480 kcal/mol
C-H 16 x 100 kcal/mol = 1,600 kcal/mol
O=O 7 x 120 kcal/mol = 840 kcal/mol
Total: 2,920 kcal/mol
Bonds Made:
C=O 14 x (- 190 kcal/mol) = - 2,660 kcal/mol
O-H 16 x (- 110 kcal/mol) = -1,760 kcal/mol
Total: -4,420 kcal/mol
Overall: 1,240 - 4,420 kcal/mol = -1,500 kcal/mol
Use the table of bond strengths to determine how much energy is released when a mol of octane is consumed.
CH3CH2CH2CH2CH2CH2CH2CH3 + 12.5 O2 → 8 CO2 + 9 H2O
- Answer
-
Bonds Broken:
C-C 7 x 80 kcal/mol = 560 kcal/mol
C-H 18 x 100 kcal/mol = 1,800 kcal/mol
O=O 12.5 x 120 kcal/mol = 1,500 kcal/mol
Total: 3,860 kcal/mol
Bonds Made:
C=O 16 x (- 190 kcal/mol) = - 3,040 kcal/mol
O-H 18 x (- 110 kcal/mol) = -1,980 kcal/mol
Total: -5,020 kcal/mol
Overall: 3,860 - 5,020 kcal/mol = -1,160 kcal/mol
Given an approximate C-O bond strength of 85 kcal/mol, use the table of bond strengths to determine how much energy is released when a mol of glucose is consumed.
- Answer
-
Bonds Broken:
C-C 6 x 80 kcal/mol = 480 kcal/mol
C-H 7 x 100 kcal/mol = 700 kcal/mol
C-O 7 x 85 kcal/mol = 595 kcal/mol
O-H 5 x 110 kcal/mol = 550 kcal/mol
O=O 6 x 120 kcal/mol = 840 kcal/mol
Total: 3,165 kcal/mol
Bonds Made:
C=O 12 x (- 190 kcal/mol) = - 2,280 kcal/mol
O-H 12 x (- 110 kcal/mol) = -1,320 kcal/mol
Total: -3,600 kcal/mol
Overall: 3,165 - 3,600 kcal/mol = -435 kcal/mol
Provide a mechanism for the hydrolysis of ATP to ADP.
- Answer
-
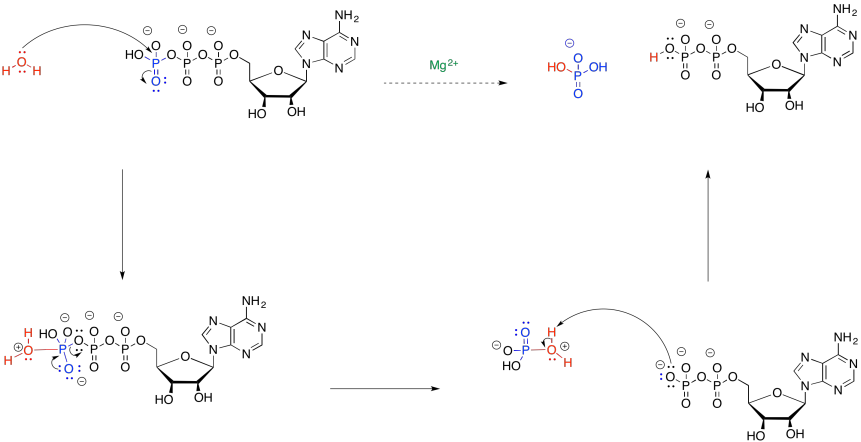
Suggest a possible role for magnesium ion in the hydrolysis of ATP.
- Answer
-
In the mechanism for hydrolysis, water acts as a nucleophile and ATP acts as an electrophile. That's a problem because ATP is negatively charged. It will not attract electrons very easily. By binding to magnesium ion (Mg2+), the charge on the ATP will be lowered, accelerating the reaction with water.


